
Abstract
Aims: Data concerning the use of bioresorbable vascular scaffolds (BVS) for chronic total occlusion (CTO) lesions are limited. The aim of this study was to evaluate the early and midterm clinical outcomes of CTO stenting with BVS.
Methods and results: Forty consecutive patients (male 78%, mean age 59.9±8.3 years, diabetics 30%) with CTO treated with BVS were enrolled. Patients with a reference vessel diameter >4 mm, metallic stents, excessive calcium and tortuosity were excluded. Mean J-CTO score was 1.6. A total of 63 BVS were implanted with an average number of 1.6 per patient, and an average scaffold length of 42.4±21.5 mm. Procedural success was achieved in all patients with no device-related complications. At follow-up (median time 556 days), there were no deaths, one patient experienced subacute and late scaffold thrombosis (ST), and another one developed symptomatic in-scaffold focal restenosis treated with repeat PCI. At control angiography, performed at a median time of 329 days in 27 patients (68%), no more restenosis or vessel reocclusion was found.
Conclusions: CTO stenting with BVS is feasible with good acute performance, and good early and midterm clinical outcomes.
Abbreviations
BVS: bioresorbable vascular scaffold
CMRI: cardiac magnetic resonance imaging
CTO: chronic total occlusion
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
LAD: left anterior descending artery
LCX: left circumflex artery
LMCA: left main coronary artery
MACE: major adverse cardiac events
MI: myocardial infarction
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
RCA: right coronary artery
ST: scaffold thrombosis
TIMI: Thrombolysis In Myocardial Infarction
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Chronic total occlusion (CTO) is currently defined as complete obstruction of a coronary artery, with a duration of at least three months, with Thrombolysis In Myocardial Infarction (TIMI) flow grade 01. CTOs are the most challenging lesions in interventional cardiology with the lowest success rate of percutaneous coronary intervention (PCI)2-4. In recent years, multiple complex techniques have been developed which have increased the success rate to above 80%5,6. After successful recanalisation of the vessel, stenting is mandatory, preferably with new-generation drug-eluting stents (DES), to ensure long-term vessel patency7,8. Nevertheless, the implantation of metallic prostheses into coronary arteries may be associated with some limitations. By caging the vessel, stents affect its vasomotion. Prolonged contact with a foreign material of first-generation DES may stimulate inflammatory and thrombotic reactions, which increases the risk of late stent thrombosis, myocardial infarction (MI) and repeat interventions9,10. Furthermore, metallic DES may hinder the non-invasive assessment of stented segments with computed tomography and exclude the possibility of future bypass graft anastomosis within these segments. There are numerous potential advantages in using bioresorbable vascular scaffolds (BVS) for such lesions: avoidance of long coronary segments being covered with metallic prostheses, restoration of endothelial function and vessel vasomotion at least within non-calcified segments, long-term favourable vessel remodelling with lumen enlargement, as well as possible non-invasive assessment of late angiographic results11-13. For these reasons, BVS have become a very promising alternative to DES. However, there are numerous potential limitations of BVS for such lesions. Severely calcified vessels may be poorly accessible for bulky devices, and their radial strength may be insufficient to resist high pressure over time14. Excessive calcium and vessel size underestimation bear the risk of major scaffold malapposition, with subsequent restenosis or thrombosis. Long subintimal channels, commonly created during PCI procedures, require multiple scaffolds, with potential aneurysm creation after complete scaffold resorption.
We report on early and midterm clinical outcomes of CTO stenting with everolimus-eluting BVS.
Methods
STUDY DESIGN, OBJECTIVES AND PATIENT SELECTION
The study was a prospective, non-randomised clinical pilot registry of patients with a CTO lesion located in a major coronary artery, treated with the everolimus-eluting BVS (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA). The main objective of the study was to assess the safety and general performance of BVS in the treatment of CTO lesions. Patients were eligible if they had symptoms and/or documented reversible ischaemia, with the presence of viable myocardium assessed by cardiac magnetic resonance imaging (CMRI). Subjects were enrolled after successful recanalisation of the target vessel, with no major complications, such as vessel perforation type >115, life-threatening arrhythmias or contrast media reactions. The following exclusion criteria were used: the presence of metallic DES in the target artery, vessel diameter >4.0 mm, excessive calcifications and/or tortuosity, long (>2 cm) subintimal channels created during the procedure, concomitant serious, life-shortening illnesses, inability to receive prolonged dual antiplatelet therapy (DAPT) or required chronic oral anticoagulation. CTO lesions were defined according to the definition of the European CTO Club1.
The study was performed according to the provisions of the Declaration of Helsinki and good clinical practice, and was approved by the local ethics committee. All patients gave written informed consent to participate.
DEVICE DESCRIPTION
The Absorb BVS consists of a backbone composed of poly-L-lactide acid, covered with an amorphous matrix, a 1:1 mixture of everolimus and poly-DL-lactide. The scaffold has a corrugated ring design with an average strut thickness of 157 microns. Two platinum markers are placed close to the proximal and distal edges of the device to allow proper positioning. The resorption process progresses gradually, mainly due to hydrolysis; thus, minimal or no inflammation is observed16,17.
STUDY PROCEDURE
All patients were pre-treated with aspirin and clopidogrel at least seven days prior to the procedure. After vessel puncture, a bolus of unfractionated heparin was given at a dose of 100 U per kilogram. Antegrade wire escalation, with the support of a microcatheter, was the prevalent technique18. After predilatation with non-compliant (NC) balloons, intracoronary nitroglycerine injections (0.1-0.3 mg) were performed. A scaffold size was selected according to online QCA, with an in-segment maximal lumen diameter (Dmax) used as reference. The BVS was deployed gradually, with 2 atm increments every five seconds. Scaffold post-dilatation with an NC balloon, 0.25-0.5 mm larger than the nominal scaffold diameter, was strongly recommended. In cases where multiple scaffolds were needed, a scaffold-to-scaffold or marker-to-marker technique was used to avoid or minimise the length of overlapping segment. The procedure was regarded successful if final TIMI 3 flow was obtained and final diameter stenosis was below 30% by visual estimation. Intravascular ultrasound (IVUS) or optical coherence tomography (OCT) was used at the discretion of the operator. On discharge, all patients were instructed to remain on DAPT for 12 months, and then lifelong on aspirin alone. Clopidogrel was used until March 2014, when it was replaced by ticagrelor for at least three months post procedure.
QUANTITATIVE CORONARY ANALYSIS
In each patient, after lesion predilatation and nitroglycerine injection, online reference vessel diameter and Dmax were calculated to size the scaffolds. Detailed offline 3D QCA analysis was performed using dedicated software (QAngio XA 3D RE straight; Medis, Leiden, The Netherlands). Reference vessel diameter, minimal luminal diameter (MLD), minimal luminal area (MLA) and percentage diameter stenosis (DS%) in-scaffold and in-segment were calculated based on two orthogonal projections post procedure. At follow-up, the same measurements in the same projections were obtained. Restenosis was defined as in-scaffold or in-segment luminal diameter stenosis >50%.
OPTICAL COHERENCE TOMOGRAPHY ANALYSIS
Optical frequency domain imaging (OFDI) (LUNAWAVE®; Terumo Corp., Tokyo, Japan) was performed in selected patients at baseline and at follow-up. OCT images were analysed offline using dedicated software (QIvus® 3.0; Medis). Quantitative analyses were performed according to BVS-dedicated methods19 by detection of lumen and stent diameters and areas; the presence of edge dissections and tissue protrusions at baseline, and the percentage of malapposed struts and uncovered struts at follow-up were calculated.
STUDY ENDPOINTS AND DEFINITIONS
Device feasibility and safety were the key endpoints of the study. The clinical endpoint was a target vessel failure (TVF), defined as the combination of cardiac death, target vessel MI, or clinically driven target vessel revascularisation (TVR). The main procedural outcomes were device success (successful device deployment at the intended segment), and procedure success rate (residual stenosis <30% and TIMI flow grade 3, with no major procedural complications).
The secondary outcome of interest was the incidence of scaffold thrombosis (ST), classified according to the Academic Research Consortium criteria20. Both periprocedural and spontaneous MI were defined according to the third universal definition21.
STATISTICAL ANALYSIS
A standard descriptive statistic was applied in the analysis. All continuous variables are presented as means±standard deviation or medians and interquartile range (IQR). Categorical variables are presented as counts and percentages or frequencies. The Kaplan-Meier (K-M) method was used to calculate the event rates at follow-up.
Results
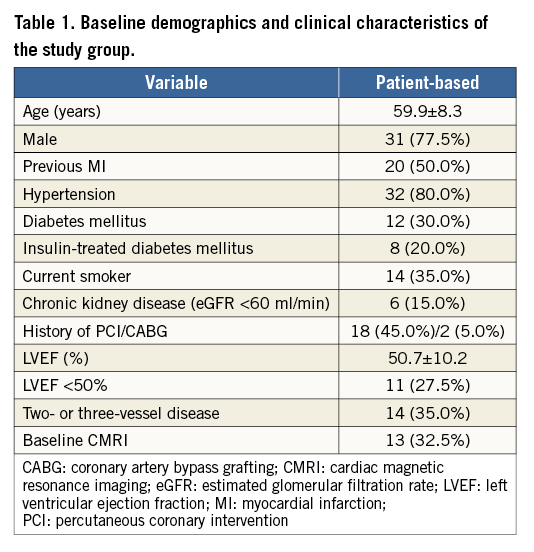
PATIENT POPULATION AND LESION CHARACTERISTICS
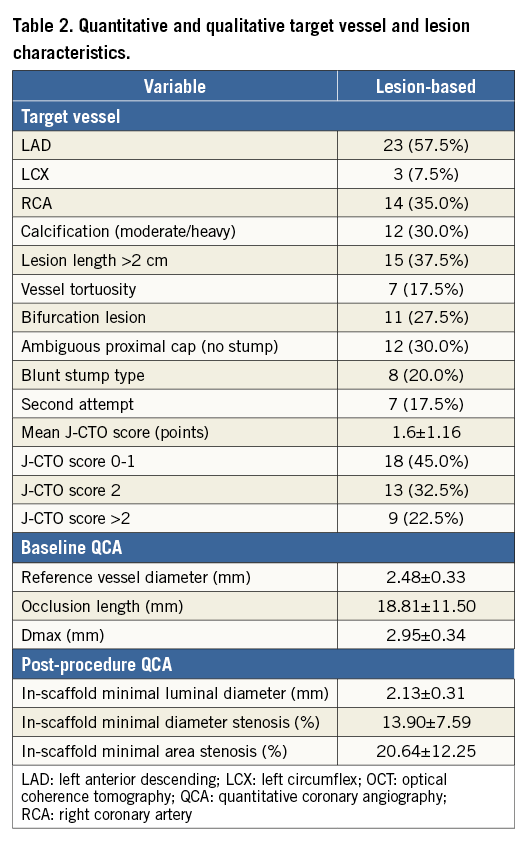
Between March 2013 and October 2014, 105 patients with CTO lesions were treated in our institution, of whom forty met all inclusion/exclusion criteria. The following exclusion criteria were found: unsuccessful wire passage to distal lumen (23 patients), major complication (two patients), long subintimal channel (seven patients), excessive calcium or tortuosity (12 patients), vessel reference diameter >4.0 mm (five patients), serious life-shortening illness (two patients), chronic oral anticoagulation (nine patients), potential problem with prolonged DAPT (five patients). None of the study patients received metallic stents during PCI. The baseline demographic and clinical characteristics are presented in Table 1. The mean patient age was 59.9±8.3 years, 31 were males (77.5%), and 12 had diabetes mellitus (30%). Table 2 summarises vessel and lesion characteristics. The left anterior descending artery (LAD) was the culprit vessel in 23 patients (57.5%), and the right coronary artery (RCA) in 14 (35.0%). According to the Multicenter CTO Registry in Japan (J-CTO) classification22, 18 lesions (45.0%) were classified as easy or intermediate, 13 as difficult (32.5%) and nine as very difficult (22.5%). On QCA, mean proximal and distal reference vessel diameters were 2.97±0.28 mm and 2.36±0.27 mm, respectively, and the mean lesion length was 18.81±11.50 mm.
Procedural details
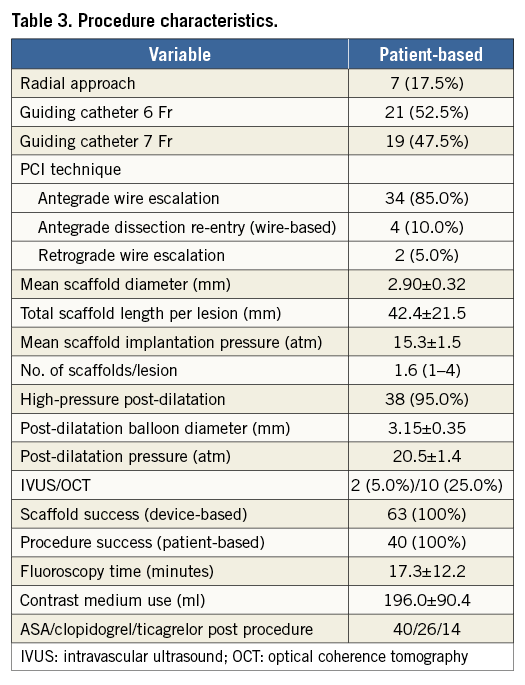
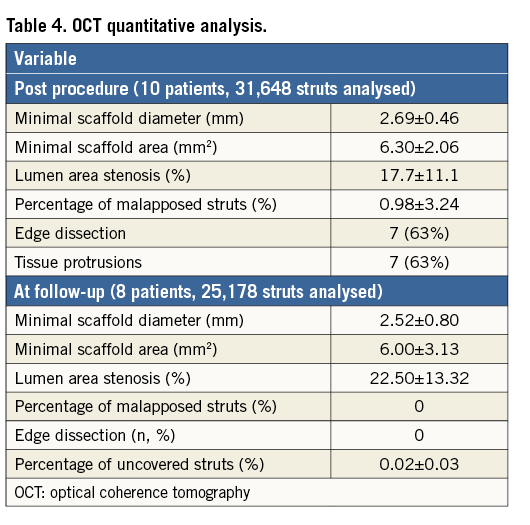
Complete procedural data are presented in Table 3. The antegrade approach was used in 38 patients (95%), and the retrograde, after failed antegrade in the same session, in the remaining two (5%). In total, 63 scaffolds were implanted with an average number per lesion of 1.6. The device and procedure success rates were 100%. Mean scaffold diameter and length were 2.90±0.32 mm and 42.4±21.5 mm, respectively. On QCA, the mean in-scaffold final MLD was 2.13±0.31 mm and residual stenosis 13.90±7.59% (Table 2). In OCT analysis, performed in 10 patients, the minimal in-scaffold luminal diameter was 2.69±0.46 mm, minimal luminal area 6.30±2.06 mm2, and lumen area stenosis 17.7±11.1% (Table 4).
CLINICAL OUTCOMES
In-hospital stay was clinically uneventful in all but one patient, in whom groin haematoma with a significant drop in haemoglobin was observed. Periprocedural myocardial damage was diagnosed in two subjects (asymptomatic CKMB elevation >5 times the upper limit of normal). In 26 patients clopidogrel was prescribed for twelve months, whereas in the remaining 14 ticagrelor was recommended for at least three months, followed by clopidogrel. At 30 days there were no deaths, but one patient developed subacute ST-segment elevation MI due to ST at five days post procedure. The patient was a 59-year-old man, current smoker with prior myocardial infarction and EF of 45%. During the index procedure, a CTO located in a mid portion of the LAD was opened and covered with two scaffolds, both 2.5×28 mm. Due to diffuse disease in the proximal part of the same vessel, an additional 3.0×28 mm scaffold was implanted during the index procedure (Figure 1A, Figure 1B). The patient was discharged on clopidogrel and aspirin. Five days later he was admitted to another hospital with anterior ST-segment elevation MI. On angiography, mid LAD ST was diagnosed and treated with eptifibatide, thrombus aspiration and balloon angioplasty (Figure 1C, Figure 1D). Clopidogrel was replaced by prasugrel. Four months later, after switching back from prasugrel to clopidogrel, the patient experienced repeat ST with occlusion of the ostial LAD (Figure 1E, Figure 1F). He was treated with thrombus aspiration and implantation of everolimus-eluting DES, and recommended to remain on prasugrel for at least 12 months.
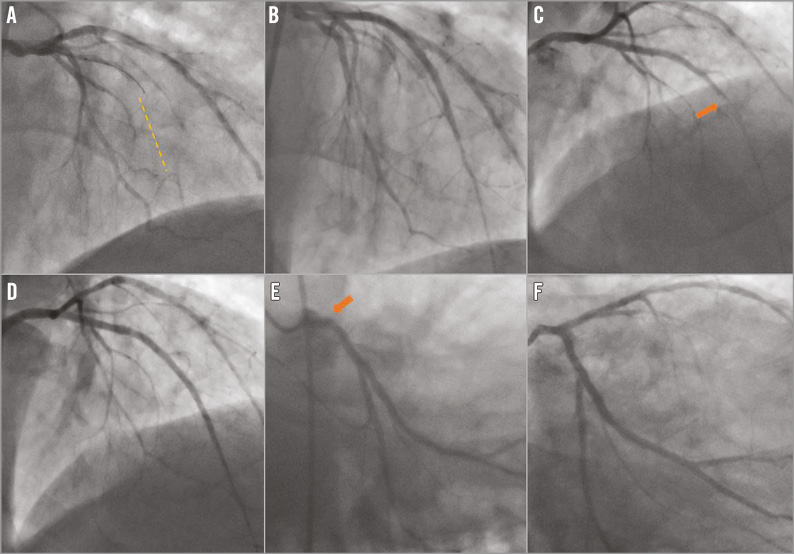
Figure 1. Serial angiograms of the patient who developed scaffold thrombosis. A) & B) Angiograms before and after the index procedure. Dashed line shows the length of the occlusion. Three 28 mm long scaffolds were implanted in proximal and mid left anterior descending artery (LAD). C) & D) Angiograms performed five days later, before and after second PCI. Mid LAD scaffold thrombosis (arrow). E) & F) Angiograms performed four months later, before and after third PCI. Repeat scaffold thrombosis at the ostium of the LAD (arrow).
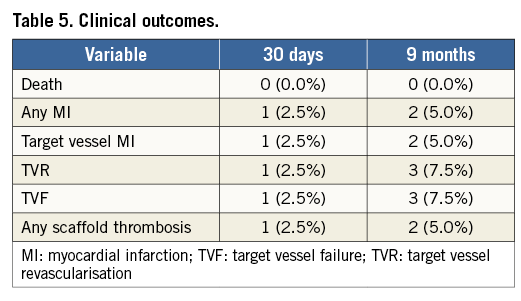
As of the end of July 2015, clinical follow-up was available at a median of 556 days (274-932, IQR 374-706), with follow-up at nine months available in all patients and at 12 months in 37 patients (92.5%) (Table 5). There were no deaths during this period of time. Two cases of myocardial infarction were observed in the same patient, as described above. One patient, a 57-year-old woman with hypertension and insulin-dependent diabetes mellitus, experienced symptomatic focal restenosis in one of the three scaffolds implanted into the RCA, treated with repeat PCI. In one patient, both aspirin and clopidogrel were prematurely stopped after four months because of gastrointestinal bleeding. No adverse cardiac events were observed in this patient, although three 3.0×28 mm scaffolds were implanted into the RCA at the index procedure. The K-M estimated cumulative incidence of TLF at 12 months was 4.9%.
ANGIOGRAPHIC AND OCT FOLLOW-UP
Control coronary angiography was performed in 27 patients (68%) at a median time of 329 days (57-680 days, IQR 172-415 days). There were no cases of vessel reocclusion. Detailed QCA analysis is presented in Table 6.
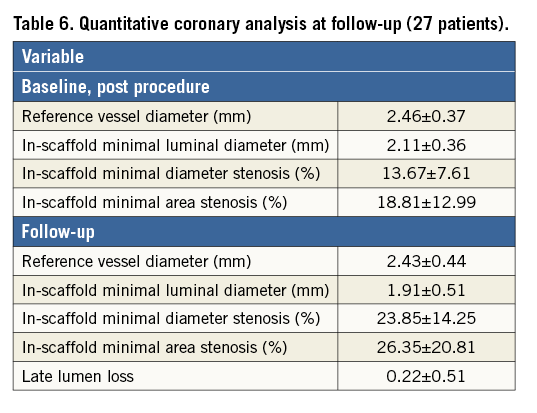
In eight patients OCT was performed at follow-up at a mean time of 422 days. Scaffold struts were completely covered with neointima in all but one patient, in whom after 10 months some uncovered struts were found in the proximal segments of the vessel (Figure 2). Detailed quantitative OCT analysis is presented in Table 4.
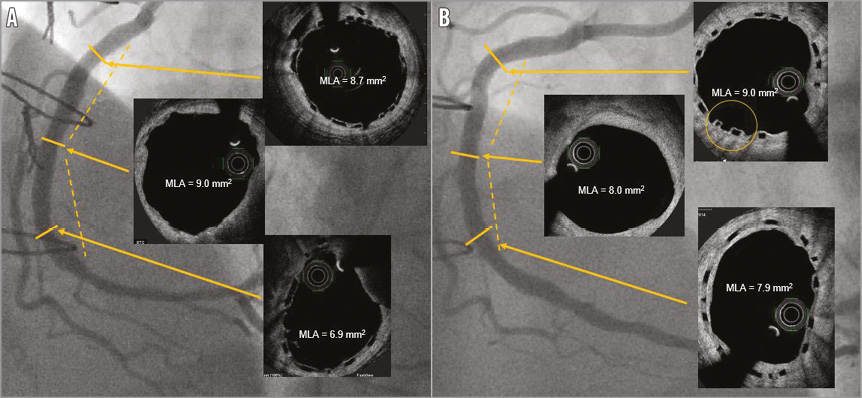
Figure 2. Right coronary artery after implantation of two 3.5×28 mm scaffolds. A) An angiogram and OCT images after baseline procedure. Scaffold underexpansion in the distal segment. Gap between scaffolds in the mid RCA. B) An angiogram and OCT images 10 months post procedure. Increase in the luminal area in the distal segment, small decrease in the luminal area in the gap between scaffolds, possibly uncovered struts in the proximal segment (circle).
Discussion
We reported on the early and midterm clinical outcomes of forty patients with CTO lesions treated with the implantation of the Absorb BVS. In this selected population with relatively less complex CTO lesions, in-hospital results were good with a high device success rate and no device-related complications. Early results were also good, with one case of subacute ST five days post procedure. This patient represents a typical case of resistance to clopidogrel, confirmed by repeat ST four months later after switching back from prasugrel to clopidogrel.
CURRENT CLINICAL EXPERIENCE WITH BVS
The current design of the Absorb BVS has been evaluated in multiple trials, mostly with low numbers of patients and relatively simple lesions. None of them was dedicated to CTO. A relatively more complex population has been studied in the ABSORB EXTEND registry. The interim observation of the first 512 patients revealed low rates of MACE and TVF, 4.3% and 4.9%, respectively. The cumulative rate of definite and probable ST was 0.8%23. ABSORB II was the first study comparing BVS with its everolimus-eluting metallic counterpart, XIENCE (Abbott Vascular)24. In this multicentre randomised trial, 501 patients with one or two de novo simple lesions were enrolled. The twelve-month, device-orientated composite endpoint was similar for BVS and DES (5% vs. 3%, p=0.35). Three patients in the BVS group developed ST. The only study reporting on “real-world” patients is the recently published GHOST-EU registry25. A total of 1,189 patients were enrolled, including 93 subjects with CTO. At six months, the rate of cardiac death was 1.0%, target vessel myocardial infarction 2.0%, TLR 2.5%, and TVR 4.0%. The cumulative incidence of definite/probable ST was 1.5% at 30 days and 2.1% at six months.
Recently, Vaquerizo et al published preliminary results of 35 patients treated with BVS implantation for CTO lesions26. All patients were followed with multislice computed tomography. Patients’ demographic and clinical characteristics were similar to our group, except for diabetes mellitus, which was more prevalent in our patients (20% vs. 30%). Unlike our results, in the Spanish series there was no ST, but two cases of in-scaffold vessel reocclusion were identified.
THE RISK OF SCAFFOLD THROMBOSIS
Two major issues should be addressed regarding ST, the learning curve and appropriate antiplatelet therapy. Over time, we modified the BVS implantation technique by increasing the rate of high-pressure scaffold post-dilatation, which is currently performed in almost every patient, with the use of a non-compliant balloon, 0.25-0.5 mm larger than the scaffold. We are in favour of scaffold sizing according to maximal reference diameter, which stays in line with the current practice of major European centres27. Based on our experience, we decided to modify antiplatelet treatment after BVS implantations. Currently, we recommend ticagrelor instead of clopidogrel for at least three months post procedure, and in complex cases with multiple scaffold implantations for at least twelve months. We believe that both implantation technique and optimal DAPT significantly contribute to improving the safety of PCI with the use of current scaffolds.
Study limitations
The major limitation of the study is its non-randomised, single-centre design with a small number of patients. Patient selection took place, excluding those with unsuccessful wire passage to distal lumen and/or unfavourable lesion anatomy. Finally, a longer observation time may be needed to capture all potential adverse events related to the procedure.
Conclusions
In a selected CTO population, stenting with bioresorbable everolimus-eluting scaffolds is feasible with good acute performance and good early and long-term clinical outcomes. An adequate stenting technique and optimal DAPT are of crucial importance. The results of our study represent a major step forward towards more complete implementation of BVS in coronary interventions.
| Impact on daily practice Our results indicate the feasibility and some potential risks of using BVS in selected patients with relatively less complex CTO lesions. In such lesions, where long coronary segments are usually covered with stents, the replacement of “full metal jacket” with “full polymer jacket” may be particularly encouraging. Nevertheless, in severely calcified CTO lesions the benefit of endothelial function and vessel vasomotion restoration may be lost. |
Conflict of interest statement
M. Lesiak has received payments as an individual for advisory board and speaker’s honoraria from Abbott Vascular, AstraZeneca, B. Braun, Biotronik, Boston Scientific, and Tryton Medical. S. Grajek has received payments as an individual for advisory board and speaker’s honoraria from AstraZeneca, Servier, Pfizer, Sandoz, Adamed, and Polpharma. A. Araszkiewicz has received payments as an individual for advisory board and speaker’s honoraria from Abbott Vascular. P. Mitkowski has received payments as an individual for advisory board and speaker’s honoraria from Boehringer Ingelheim, Boston Scientific, Biotronik, Gilead, HAMMERmed, Medtronic, Pfizer, St. Jude Medical, and Servier. The other authors have no conflicts of interest to declare.

