Abstract
Aims: We studied a novel sirolimus eluting stent with a bioabsorbable coating (SES) in >3months old chronic total coronary occlusions (CTO).
Methods and results: Ninety-five patients were randomised to either BMS (n=47) or SES (n=48). The primary endpoints were late lumen loss (LLL) and in-segment restenosis (ISR) after six months. Secondary endpoints were target vessel revascularisation after six and 24 months. Occlusion length (37.6 mm), reference diameter (2.8 mm) as well as the stented segment (45.5 mm) were similar in both groups. Up until six months no death, myocardial infarction or stent thrombosis occurred in either group. Angiographic follow-up (45BMS/46SES): LLL 1.8 mm/0.77 mm (p<0.0001), ISR 60%/17.4% (p<0.0001). In-segment re-occlusion 15.5%/0% and TVR 53.3%/10.8% (p<0.0001). After 24 months: 1 BMS, 2 SES patients died; 0 BMS, 0 SES infarction; 0 BMS, 0 SES stent thrombosis and 3 BMS, 0 SES TVR between six and 24 months. Thus total TVR after 24 months was 60% for BMS and 10.8% for SES (p<0.0001).
Conclusions: The alternative sirolimus-coated stent was shown to reduce the relative risk of restenosis after six months by 71%, and of TVR after 24 months by 82%. Between six and 24 months, neither stent thrombosis occurred, nor repeat revascularisation was required in patients who received a SES.
Introduction
Chronic total occlusions (CTO) present with no antegrade filling of the distal vessel and have angiographic or clinical evidence of an occlusion duration of more or equal to three months1. One-third of all significant coronary stenoses at angiography are chronic total occlusions (CTO), but only 10-15% of them are treated with percutaneous coronary intervention (PCI)2. Noteworthy, reopening of CTO has been shown not only to alleviate anginal symptoms, but also to improve left ventricular ejection fraction, decrease the need for coronary bypass graft surgery (CABG) and improve long-term survival3-7. Although several randomised trials demonstrated the efficacy of stent implantation over balloon-only angioplasty, even with stents there remains a significant rate of both restenosis (32% to 55%) and re-occlusion (8% to 12%)8-10. Several non-randomised and one randomised trial in CTO patients showed that drug eluting stents might be able to cut restenosis by 72-93% and re-occlusion by 90-100% when compared to bare metal stents11-14. In the only randomised trial (PRISON II) a true chronic total occlusion was present only in 45% of the 200 patients. In this subgroup, the restenosis rate for the sirolimus-eluting stent with a non-degradable polymer coating was 7%, and the re-occlusion rate was 5% as compared with 39% and 15% respectively for bare metal stents14.
The main disadvantage of the current sirolimus-eluting stent available in Europe is the strut thickness of 140 µm and a non-absorbable 12 µm polymer coating that may contribute to delayed endothelialisation and thus increased risk of stent thrombosis15. In 2002, a stainless steel stent with a biodegradable polymer coating of 4 µm to allow controlled release of sirolimus (SES) was developed and evaluated in several animal trials (the CORACTO® stent). The aim of our study was to prove the safety and effectiveness (with regard to in-segment restenosis and target lesion revascularisation) of this SES.
Patients and methods
The study was conducted with ethical committee approval in accordance with the declaration of Helsinki at two investigative sites and was registered as DE/CA 25/00000365-00 (DIMDI). Both stents were of stainless steel (Constant®) with a strut thickness of 90-100 µm. The drug eluting stent was identical, but contained sirolimus 170 µg/cm2 embedded in a flexible and expandable bioabsorbable coating (thickness 3-4 µm). (Figure 1)
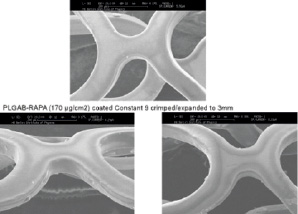
Figure 1. Both stents were of stainless steel with a strut thickness of 100 µm. The drug eluting stent contained sirolimus 170 µg/cm2 embedded in a flexible and expandable bioabsorbable coating (thickness 3-4 µm).
In a pig-model, the SES showed a complete endothelialisation after 28 days with no signs of inflammation. (Figure 2)
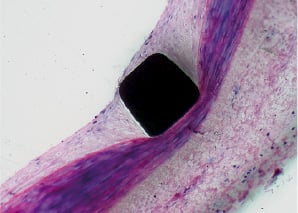
Figure 2. In a pig model, the SES showed a complete endothelialisation after 28 days with no signs of inflammation.
Inclusion and exclusion criteria
Patients were considered for the study if they met all of the following inclusion criteria and none of the exclusion criteria.
INCLUSION CRITERIA
– The patient must be eligible to undergo planned treatment of one or more lesions in a chronically occluded native coronary artery (CTO). CTO was defined as: intra-segment TIMI 0 flow despite vigorous injection and clinical evidence or high likelihood of occlusion age not less than three months.
– The patient or legal guardian must have signed the informed consent form.
– The patient agrees to return at one, three, six and nine months for an office visit to assess cardiovascular status.
– He/she should be willing to have a angiographic follow-up at nine month, although denying this was not an exclusion criterion.
– Patient must be able to take aspirin and clopidogrel or ticlopidine.
EXCLUSION CRITERIA
– Age less than 18 years old.
– The patient has history of bleeding diathesis or coagulopathy.
– The patient is simultaneously participating in another investigative interventional cardiovascular device or drug study.
– The patient must have completed the follow-up phase of any previous study at least 30 days prior to enrolment in this study.
– Known hypersensitivity or contraindication to aspirin, clopidogrel or stainless steel, as well as a sensitivity to contrast dye that, in the opinion of the investigator, cannot be adequately pre-medicated.
– Women of child-bearing potential.
– The patient has a poor six months prognosis due to noncardiac disease (e.g., late-stage cancer).
– Lesion could not be crossed with a balloon.
– Target vessel reference diameter <2.5 mm or >4.5 mm.
Between August 2003 and December 2004, 95 non-consecutive patients with CTO (including 16 with functional occlusion according to blinded QCA evaluation) had been enrolled in our study and received either a bare metal stent (BMS, n=47) or a sirolimus-eluting stent (SES, n=48). Only stents of 17 mm length were used. A total of 253 stents were implanted (126 BMS and 138 SES). All patients were pretreated with 300 mg clopidogrel and 500 mg aspirin before intervention. Dual platelet inhibition with clopidogrel 75 mg/day and aspirin 100 mg/day was given for six months followed by aspirin 100 mg/day.
The occlusion age determined by prior angiogram, history of infarction or as estimated according to clinical history was at least three months. The mean duration which could be determined was 16 months in the BMS group and 19 months in the DES group, respectively.
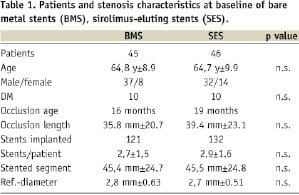
Patients in both groups showed no significant difference regarding age, gender or diabetes mellitus (Table 1). The lesions in both groups showed no significant difference regarding occlusion age, occlusion length, reference diameter or length of stented segment (Table 1).
Primary endpoints were defined as angiographic in-segment late lumen loss (LLL) and in-segment restenosis (ISR, angiographic stenosis at six months follow-up >50%). Secondary endpoints were clinically driven target vessel revascularisation (angiographic restenosis and typical symptoms/ischaemia or re-narrowing >70%) after six, 12 and 24 months.
Repeat TLR was defined as clinically or angiographically driven revascularisation of in-stent restenosis, defined as restenosis within a stent or within 5 mm of the stent borders. Repeat TVR was defined as clinically or angiographically driven revascularisation of a stenosis beyond 5 mm of the stent borders.
All events a well as the coronary angiograms were blinded before evaluation by a physician otherwise not involved in the study (A. Rabe).
Quantitative coronary angiography
Coronary angiograms were digitally recorded at baseline, immediately after the procedure, as well as at 6-month follow-up and were assessed offline at the quantitative angiographic core laboratory of the Main Taunus Klinikum Bad Soden with an automatic edge detection system16 by a physician (A. Rabe) who was not provided with any clinical information on the type of stent used. Although the two operators at the two sites included total occlusions only, the offline QCA analysis revealed that in 16% of the patients, in at least one view, an intracoronary channel was detectable.
Statistics
To show improvement in angiographic percent diameter stenosis from the expected 35% to 20% (@p<0.05), the study required a minimum of 32 evaluable patients per group.
Continuous variables are reported as mean and standard deviation and effects across the two groups were analysed by analysis of variance. Dichotomous variables were reported in percent. Group-wise comparisons were performed with a Pearson’s chi-square test or Fisher’s exact test. All comparisons were performed on an intention-to-treat basis using all patients available with follow-up analysis.
Results
No death, myocardial infarction or stent thrombosis occurred in the first six months in either group. After six months, 91 out of 95 patients (with 253 of the 264 implanted stents) were available for angiographic follow-up (45 patients with 121 BMS and 46 patients with 132 SES). After 24 months, none of the patients experienced a myocardial infarction, neither was a CABG operation necessary. One patient in the BMS group and two patients in the SES group died, none related to the target vessel. Typical angiograms of two patients from both groups are shown in Figures 3 and 4.
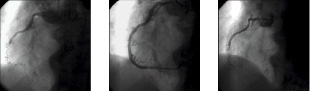
Figure 3. CTO of RCA before and after stenting with a BMS and at follow-up.
Figure 4. CTO of RCA before and after stenting with a SES and at follow-up.
Primary endpoints
Late lumen loss (LLL) was 1.8 mm±0.82 for BMS and 0.77 mm±0.63 for SES (p<0.0001).
The mean follow-up stenosis was 66.5%±27.3 (BMS) versus 31.1%±22 (SES) (p<0.0001).
In-segment restenosis, defined as restenosis within the stented segment including 5 mm beyond the stent edges, occurred in 27 of 45 patients treated with BMS (60%) and in eight of 46 patients treated with SES (17.4%) (p<0.0001). In-segment re-occlusion was seen in seven of 45 patients with BMS (15.5%) and in none of the patients with SES. Restenosis according to the Mehran classification17 was also more exaggerated in the uncoated stent group: BMS vs. SES showed in-stent restenosis class 1 in 5 vs. 6, class 2 in 5 vs. 0, class 3 in 10 vs. 2 and class 4 in 7 vs. 0 patients. Restenosis occurred at the border of two stents i.e., the area of possible “stent-overlap” in 54% (19 of 35 cases). It reached 63% after BMS and 30% after SES of the respective patients demonstrating restenosis.
Secondary endpoints
After six months, target vessel revascularisation (TVR) was necessary in 24 of 45 patients with BMS (53.3%) and five of 46 patients with SES (10.8%) (p<0.0001); 24 month follow-up was available in 81 of 91 patients (89%), BMS 87%, SES 91%. Three patients with BMS and none with SES needed repeated TVR between six and 24 months, resulting in a 60% total-TVR in the BMS group and 10.8% with SES (p<0.0001). The overall risk reduction of restenosis with SES as compared with BMS after six months was 71%, and the relative risk reduction of TVR after 24 months was 82%. (Table 2) (Figures 5 and 6)
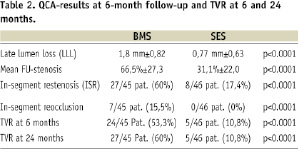
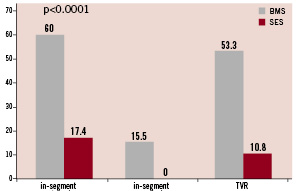
Figure 5. In-segment restenosis, re-occlusion and TVR at 6-month follow-up.
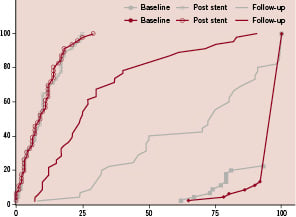
Figure 6. Cumulative frequency of in-segment stenosis at baseline, post-PCI and at follow-up shows sufficient inhibition of proliferation with high likelihood of complete endothelialisation at six months. (LLL 0.7 vs. 1.8).
Discussion
This is the first randomised trial of chronic total occlusions aged >3 months treated with bare metal stents or drug eluting stents. In the Prison II trial comparing a sirolimus-eluting stent (SES) with a non-degradable polymer (Cypher®; Cordis, Johnson & Johnson, Warren, NJ, USA) with a bare metal stent in total occlusions, the mean occlusion age was 2.8 months and only 45% of the patients met the criteria of CTO14. In this trial, the SES reduced restenosis from 41% to 11% in occlusions of about 16 mm length and TVR for BMS was necessary in only 22%, underlining a relatively low complexity of disease. It is well known that restenosis after stenting is negatively influenced by lesion length, stent length18, vessel diameter and diabetes and for bare metal stents in long chronic occlusions may well exceed 50%19-21. In the CORACTO trial, vessel diameter and the presence of diabetes are similar to those in the PRISON trial, however occlusions are considerably longer (36-39 mm). As an evidence of complexity, restenosis was reduced from 60% to 17.4%, and TVR in the BMS group was necessary in 53.3%. These findings are further confirmed by the late lumen loss of 1.0 mm versus 0.05 mm in the Prison trial and 1.8 mm and 0.77 in the CORACTO trial – i.e., a difference in lumen size at follow-up between BMS and DES of 0.95 mm (PRISON trial) and 1.3 mm (CORACTO trial). In the PRISON trial, after three years, none of the patients in the BMS group experienced stent thrombosis, while it occurred in 5% of the 100 patients receiving SES22. Although clopidogrel was stopped after six months and no stent thrombosis was witnessed within 24 months in the group of 48 patients who had received 138 SES in the CORACTO trial, the number may be too low to draw the conclusion that the biodegradable coating is safer than the current non-degradable polymer-coating.
Conclusion
The CORACTO® stent, with an alternative thin biodegradable sirolimus-coating, has proven superior to a similar bare metal stent in patients with the worst coronary lesions (CTO). It reduced the restenosis after six months by 71% and TVR after 24 months by 80% when compared with the control group. These results favourably compare with the PRISON II data where the reduction of restenosis by a sirolimus-eluting stent was 73%14. The novel bioabsorbable coating appears safe and effective. Despite a dual platelet inhibition of six months only, followed by ASS, no stent thrombosis occurred.

