
Abstract
Aims: The PRISON IV trial investigated the next-generation sirolimus-eluting stent (SES) with ultra-thin struts and biodegradable polymer against the second-generation everolimus-eluting stent (EES) with thin struts and durable polymer in patients with successfully recanalised chronic total occlusions (CTO). In this study, we examined the secondary optical coherence tomography endpoints.
Methods and results: The main PRISON IV trial randomised 330 patients to either SES or EES. At nine months, 281 (85%) patients underwent repeat angiography. Of these, 60 consecutive patients received optical coherence tomography divided over both stent groups. The mean number of struts analysed was 750±337 and 633±358 in SES and EES patients, respectively (p=0.07). The minimal lumen area, minimal stent area, maximal neointima area and neointimal thickness were comparable between the groups (4.8±2.1 and 4.4±1.5 mm2; 5.3±1.8 and 5.3±1.4 mm2; 2.5±2.0 and 2.2±1.5 mm2; 0.7±1.7 and 0.4±0.2 mm). The percentage of uncovered struts was higher with EES (6.2±7.5% and 11.9±13.4%, p=0.04), whereas the percentage of malapposed struts and mean number of coronary evaginations were significantly higher with SES (2.9±4.0% and 1.2±2.4%, p=0.02; 18.5±17.7 and 5.3±3.1, p=0.004).
Conclusions: The optical coherence tomography findings of this substudy demonstrated improved strut coverage with ultra-thin strut SES with bioresorbable polymer compared to thin-strut EES with durable polymer in CTO. On the other hand, SES showed a higher rate of stent strut malappositon and coronary evaginations. The clinical relevance of these findings remains to be demonstrated.
Abbreviations
CTO: chronic total occlusion(s)
EES: everolimus-eluting stent
MACE: major adverse cardiac events
MI: myocardial infarction
OCT: optical coherence tomography
SES: sirolimus-eluting stent
TIMI: Thrombolysis In Myocardial Infarction
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Percutaneous revascularisations of chronic total occlusions (CTO) have become a feasible alternative to surgical treatment with fast-developing recanalisation techniques, operator expertise and drug-eluting stent (DES) technology, resulting in high rates of procedural success. Despite improved treatment efficacy with drug-eluting stents, patients remain at higher risk of stent thrombosis and restenosis compared to non-CTO percutaneous coronary interventions (PCIs)1. Stent strut malapposition, in-stent neoatherosclerosis, uncovered struts and stent underexpansion have been identified as causal mechanisms of very late stent thrombosis2. Chronic total occlusions are more prone to impaired coronary healing after stenting for several reasons: hard fibrocalcific lesions with large plaque burden need long stented segments with multiple overlapping stent strut layers. In the PRISON IV trial, the next-generation sirolimus-eluting stent with ultra-thin struts and biodegradable polymer (SES) was compared to a second-generation everolimus-eluting stent with thin struts and durable polymer (EES) in patients with successfully recanalised CTO. In this study, we investigated coronary healing at nine months after stent implantation with optical coherence tomography.
Methods
STUDY OVERVIEW AND ENROLMENT
The PRISON IV prospective, randomised, multicentre, single-blinded, non-inferiority trial was conducted at eight high-volume sites in the Netherlands and Belgium. The study design and results of the primary endpoint have been published previously3. In summary, 330 patients were randomised to either SES or EES after successful CTO recanalisation. A predefined subgroup of 60 consecutive patients equally divided between both groups underwent optical coherence tomography at the time of nine-month angiographic follow-up at two investigational sites (St. Antonius Hospital, Nieuwegein, the Netherlands, and University Hospital Leuven, Leuven, Belgium). This trial is registered at ClinicalTrials.gov: NCT01516723.
ENDPOINTS
The primary endpoint was the percentage of uncovered stent struts. Secondary OCT outcomes were the percentage of malapposed stent struts, percentage of uncovered or malapposed struts, mean number of evaginations, minimal lumen area, minimal stent area, maximal neointima area and maximal thickness of neointimal coverage. OCT analyses were performed in an exploratory context with no formal sample size calculation, with no available data on stent strut coverage and malapposition for both study stents in the setting of CTO lesions when the study was designed. Angiographic and clinical endpoints and definitions were in accordance with the previously published main trial.
OPTICAL COHERENCE TOMOGRAPHY
All images were recorded with a frequency domain OCT imaging system (C7XR™ or OPTIS™ OCT imaging system; St. Jude Medical, St. Paul, MN, USA). OCT analyses were performed offline by the local core laboratory (University Hospital Leuven, Leuven, Belgium) in a blinded fashion. The OCT analysis methodology used has been described previously4. Quantitative strut level analysis was performed every third frame (0.6 mm interval) along the entire target segment. A dedicated automated software system developed at the Leuven Medical Imaging Centre was used for quantitative OCT analysis5. The threshold for malapposition was calculated as the strut thickness including polymer coating plus half of the blooming artefact (18 μm). The final cut-off value for malapposition was set at 70 and 90 μm for SES stent diameters of ≤3.0 mm and ≥3.5 mm, respectively, and 100 μm for all stent diameters of EES. Coronary evaginations were defined as the presence of an outward bulge in the luminal vessel contour between apposed struts with a depth of the bulge exceeding 250 μm, as measured from the deepest point in the bulge to the semi-automatically traced stent area.
STATISTICAL ANALYSIS
Continuous measurements were expressed in number of observations, means±standard deviation (SD) or medians and interquartile ranges (IQR). All categorical data were expressed in frequencies and percentages. Due to serious deviations from normality for many OCT parameters, comparisons between the randomised groups were made using a Wilcoxon rank-sum test or Fisher’s exact test when any of the cells had less than five patients. Individual strut probabilities for being uncovered or malapposed were analysed using a mixed logistics regression model. The model included a fixed effect for treatment and a random intercept per patient to account for within-subject correlations. Baseline characteristics and quantitative coronary analysis data were analysed, with a two-sample t-test for continuous variables, and chi-square test to compare binary and categorical outcomes. Clinical outcomes were assessed by using the Kaplan-Meier method and compared between treatment groups with the log-rank test. All tests were two-sided and assessed at a significance level of 5%. Statistical analyses were generated using SAS/STAT software, version 9.4 (TS1M3; SAS Institute, Cary, NC, USA).
Results
ENROLMENT
Between February 2012 and November 2015, a total of 117 of the 330 patients included in the study were randomised to SES or EES at two investigational sites. At nine months, follow-up angiography was available in 103 (88%) patients. Of these, 71 consecutive patients were enrolled for OCT investigation, resulting in 60 patients (SES 30, EES 30) with analysable OCT pullbacks (Figure 1).
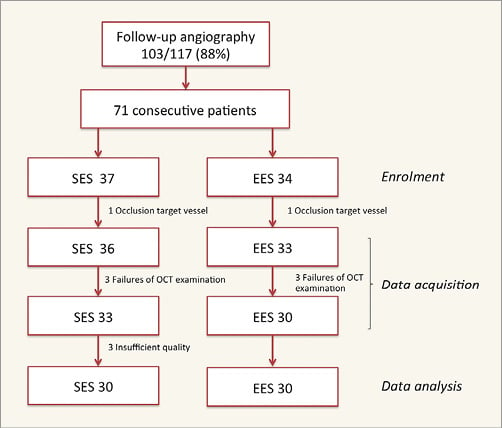
Figure 1. Study flow chart.
BASELINE CLINICAL AND PROCEDURAL CHARACTERISTICS
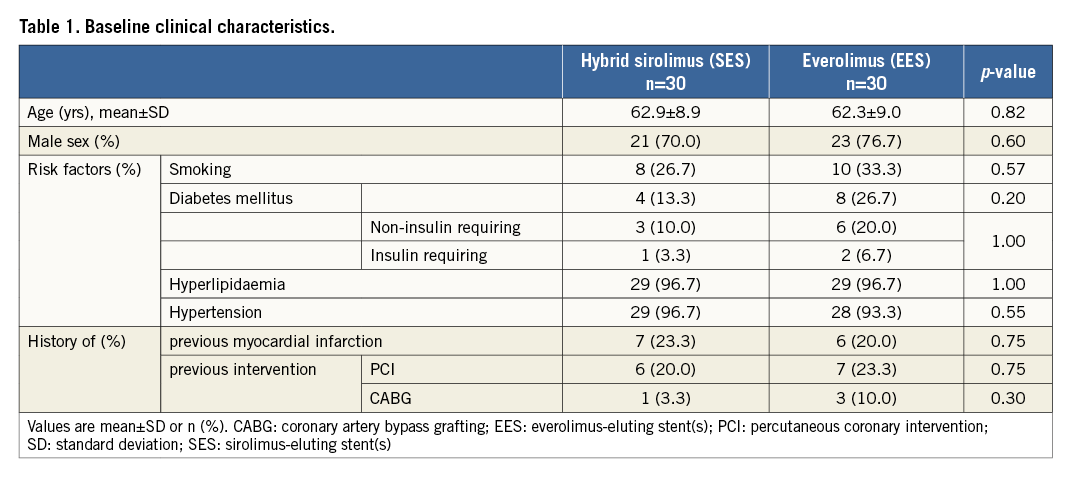
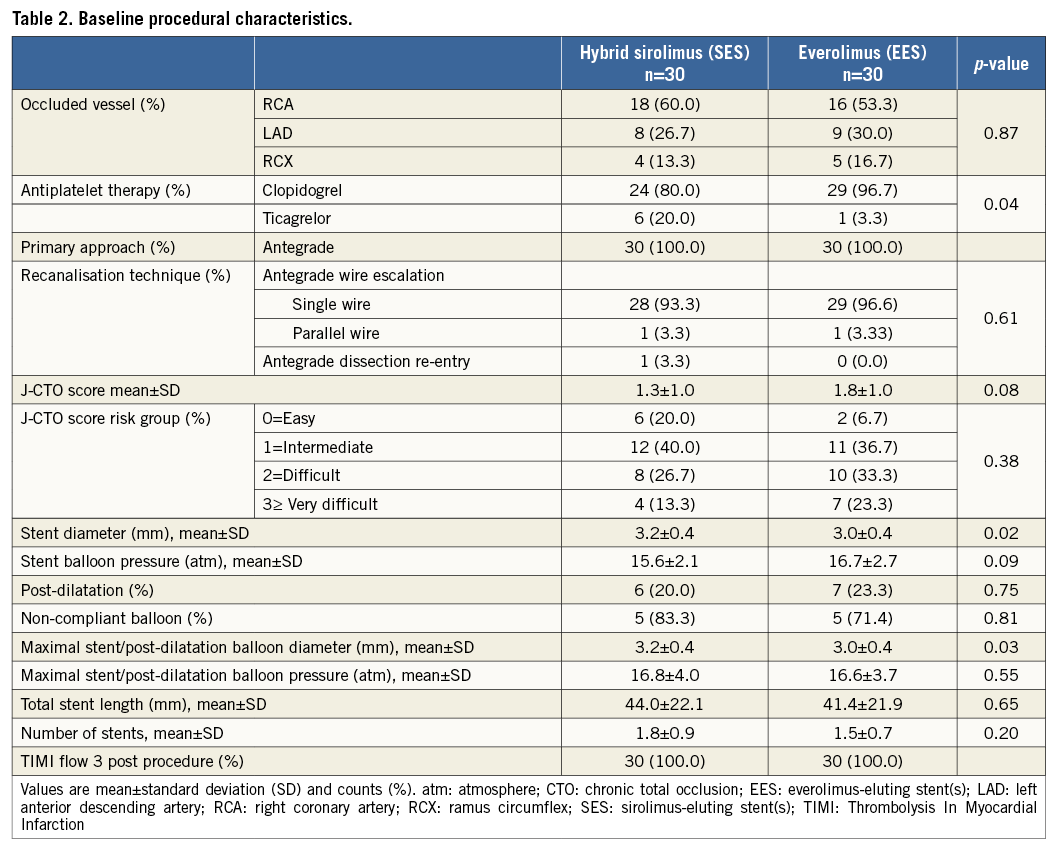
The baseline clinical and procedural characteristics were well balanced between the stent groups, except for the use of antiplatelet therapy, CTO length ≥20 mm, mean stent diameter and maximal stent/post-dilatation balloon diameter (Table 1, Table 2). All procedures were antegrade wire escalation cases and one antegrade dissection re-entry technique was used in the SES group.
OPTICAL COHERENCE TOMOGRAPHY
All analysed OCT data are shown in Table 3. The distribution of uncovered and malapposed struts is presented in Figure 2 and Figure 3. The primary endpoint demonstrated a lower percentage of uncovered stent struts with SES vs. EES (6.2±7.5% vs. 11.9±13.4%, p=0.04). The percentage of malapposed struts was higher with SES vs. EES (2.9±4.0% v. 1.2±2.4%, p=0.02) with a higher mean number of coronary evaginations (18.5±17.4 vs. 5.3±3.1, p=0.004). There were no differences in minimal lumen area, minimal stent area, maximum neointima area, or neointimal thickness. The treatment effect of SES compared to EES for individual strut data expressed in odds ratios was 0.47 (confidence interval [CI]: 0.23-0.97), p=0.04 for uncovered struts, and 2.9 (CI: 2.5-3.4), p=0.02 for malapposed struts as assessed with a mixed logistic regression model including random intercept per patient.
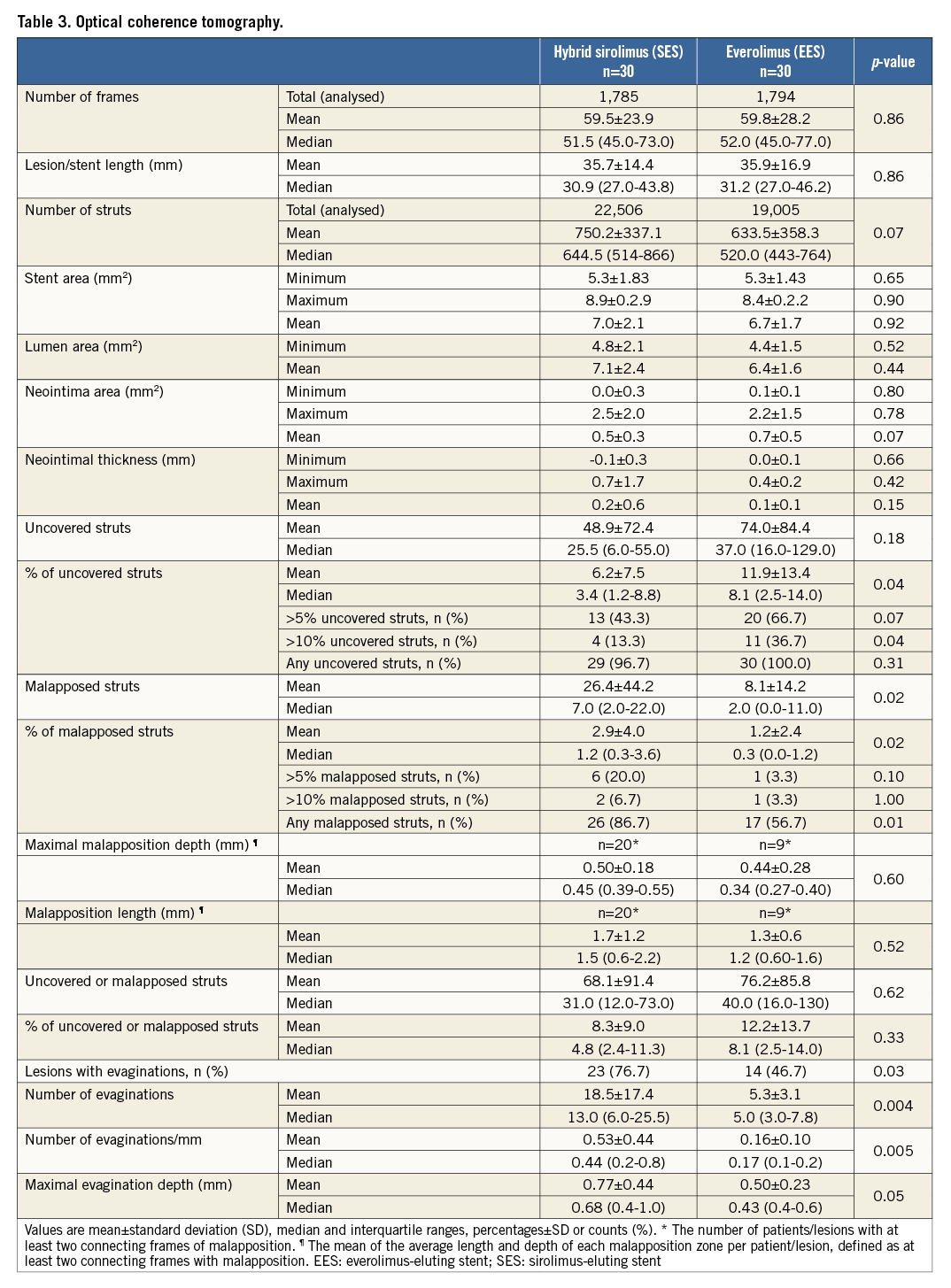
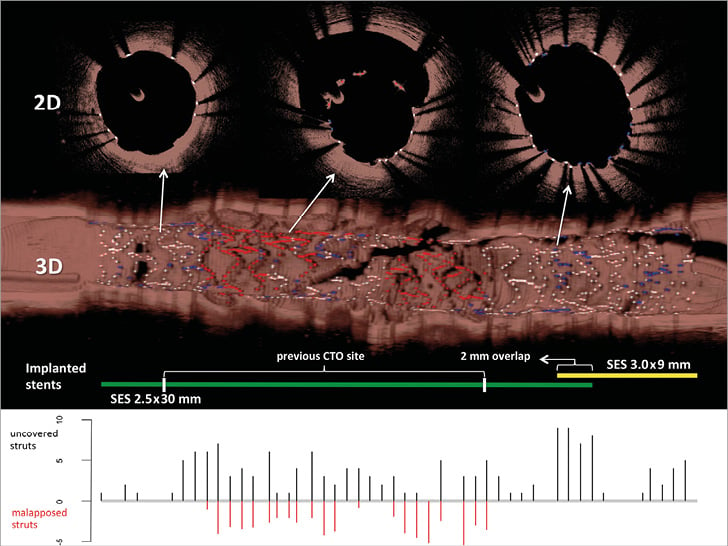
Figure 2. Uncovered and malapposed struts with 2D and 3D optical coherence tomography. The upper panels demonstrate 2D and 3D optical coherence tomography images with covered struts (white), uncovered struts (blue) and malapposed struts (red). The middle panel shows the implanted stents used with overlap zone and previous CTO site. The lower panel presents the number of uncovered (positive y-axis, black) and malapposed (negative y-axis, red) struts analysed every third frame (0.6 mm interval). 2D: two-dimensional; 3D: three-dimensional; CTO: chronic total occlusion; SES: sirolimus-eluting stent
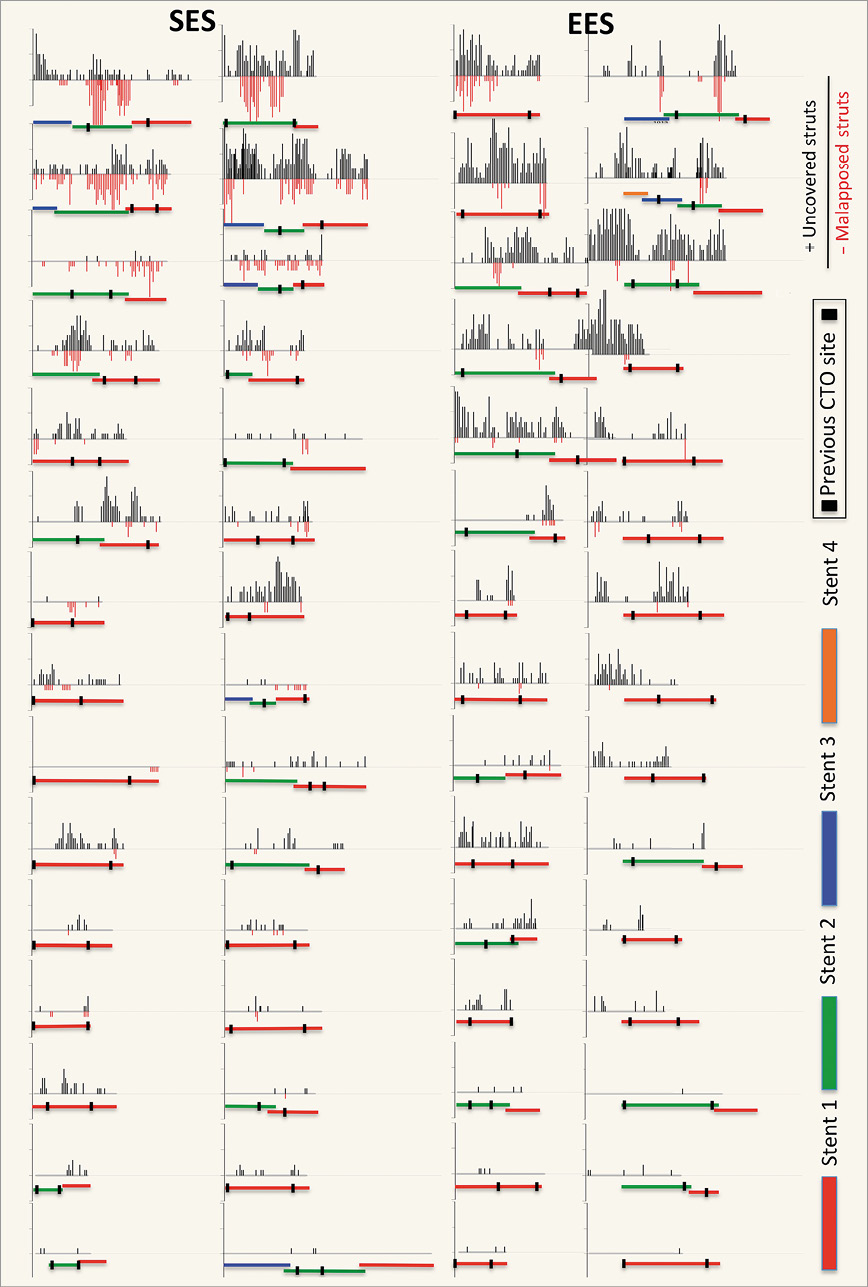
Figure 3. Distribution of uncovered and malapposed stent struts. This graph demonstrates the distribution of uncovered and malapposed struts of all patients. The number of uncovered (black) and malapposed struts (red) is reported on the positive and negative y-axis every third frame (0.6 mm interval). The implanted stents are shown from proximal (right) to distal (left) below the distribution graph with stent overlap. The previous CTO site is marked between two vertical black lines. CTO: chronic total occlusion; EES: everolimus-eluting stent; SES: sirolimus-eluting stent
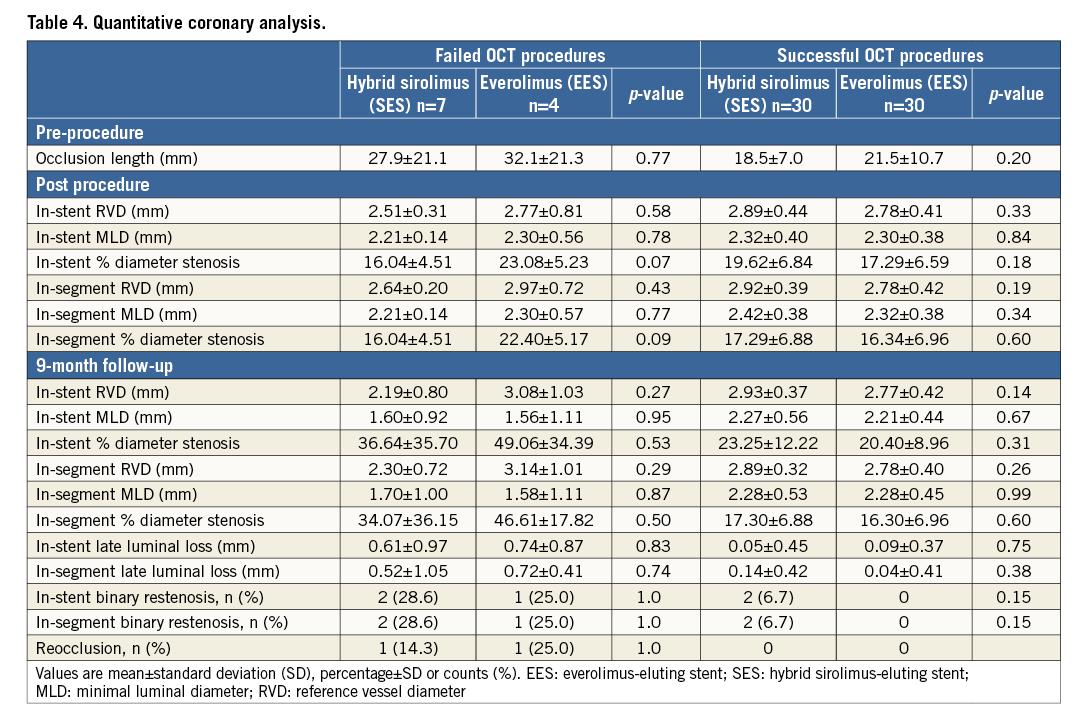
QUANTITATIVE CORONARY ANALYSIS
Angiographic analysis between baseline procedure and nine-month follow-up demonstrated no differences between the stents (Table 4). The mean occlusion length and late lumen loss were higher in patients with failed OCT procedures compared to successful OCT procedures, mainly driven by two patients with complex CTO, one in each stent group, presenting with reocclusions.
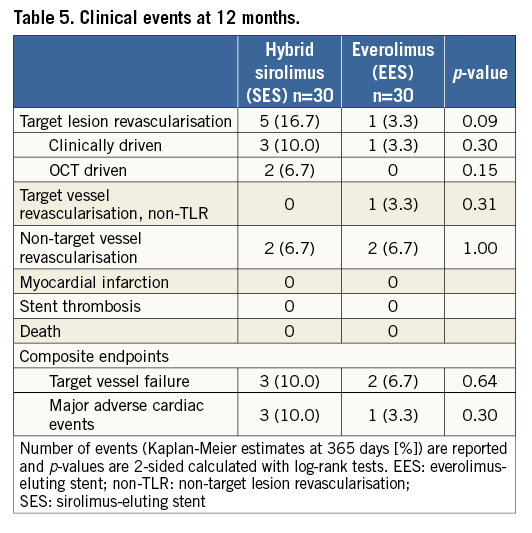
CLINICAL ENDPOINTS
Clinical events were comparable at 12 months (Table 5). Two target lesion revascularisations in the SES group were OCT driven after observing severe malappostion, treated by balloon angioplasty. No myocardial infarction, stent thrombosis or death was observed in either group, with comparable rates of target vessel failure and MACE.
Discussion
The main finding of this study is that optical coherence tomography demonstrated significantly improved strut coverage with ultra-thin strut SES with biodegradable polymer compared to thin-strut EES with durable polymer, in CTO lesions. On the other hand, SES showed a higher rate of stent strut malapposition and coronary evaginations.
This is the first study demonstrating improved stent strut coverage with the novel next-generation SES compared to second-generation EES in chronic total occlusions. The novel hybrid SES was developed with several new technical features to improve coronary healing after implantation: cobalt-chronium alloy with ultra-thin struts (60 µm) in smaller stent sizes (≤3.0 mm) to inhibit hyperplasia and thrombogenicity; amorphous silicon carbide coating to prevent hypersensitivity reactions to passive iron release; and biocompatible biodegradable polymer for drug elution to avoid inflammation and induce faster re-endothelialisation6. The EES contains thin cobalt-chromium struts (81 µm) coated with a permanent biocompatible fluoropolymer eluting everolimus. More than a decade after its introduction, the EES is considered the current gold standard in PCI with robust clinical evidence in all lesion types7.
Improved stent strut coverage observed with optical coherence tomography in the SES group reflected earlier findings in the main PRISON IV trial of higher late lumen loss with SES compared to EES3. In-stent minimal lumen area, maximal neointimal area and neointimal thickness were not different between the stents. Importantly, two consecutive patients presenting with reocclusions were excluded from OCT analysis, and the average complexity of the OCT subgroup expressed by the J-CTO score was lower than the full cohort.
In the BIOFLOW II study, both study stents were investigated head-to-head in simple de novo lesions with midterm OCT follow-up available. At nine months, there was no difference in the percentage of uncovered struts and malapposed struts (1.98±3.71% vs. 2.91±3.31%, p=0.48, and 1.11±2.32% vs. 0.77±2.04%, p=0.43, with SES and EES, respectively)6. Our findings are in agreement with earlier outcomes from the ALSTER-OCT-CTO registry. In this prospective study, marked differences in coronary healing patterns were identified between treated CTO and non-CTO lesions. At follow-up, OCT demonstrated an increased percentage of uncovered and malapposed struts with the use of several new-generation DES in CTO versus non-CTO lesions8. Other published data on the everolimus-eluting stent in CTO showed significant impaired healing with high rates of uncovered and malapposed struts (uncovered struts 2.8%, CI: 2.6-2.9, and malapposed struts 9.3%, CI: 9.0-9.5). In detail, uncovered/malapposed struts were mainly noticeable in aneurysmal segments. Furthermore, a trend was shown for impaired healing in CTO treated with advanced antegrade and retrograde subintimal tracking techniques9.
To our knowledge, this is the first study reporting on coronary evaginations in treated CTO lesions. Data from our study show a higher rate of stent strut malapposition and coronary evaginations with the novel SES compared to EES. Earlier investigations in non-CTO lesions showed that coronary evaginations were highly prevalent with first-generation DES (26%) and nearly absent with second-generation EES (3%)10. Moreover, coronary evaginations were strongly associated with malapposed and uncovered struts.
In the current study, the true mechanism behind malapposition and coronary evaginations remains speculative in the absence of serial OCT investigations at both baseline and follow-up. In the SES group, two subjects received balloon angioplasty, against study protocol, after severe malapposition was observed. Furthermore, there was no use of intraprocedural IVUS or OCT guidance during the index procedures, and the rate of post-dilatation was low in both groups. Malapposition can be caused by several mechanical reasons during implantation: inadequate lesion preparation, stent sizing or post-dilatation, and biological phenomena such as biocompatibility of the stent polymer or flow-dependent late lumen enlargement in recanalised CTO. Furthermore, stent design is of major importance for optimal stent conformity to the vessel wall, with high mechanical resistance needed to prevent acute and late recoil. Both study stents have an open cell design to improve deliverability and side branch access, with three connectors and six to nine crowns. Ex vivo, bench-test results demonstrated significantly higher mechanical resistance with EES compared to SES in a direct longitudinal crush test. Nonetheless, in a clinically relevant test model, there was no difference in longitudinal crush rates between the stents11.
In real-world practice, PCI of CTO has been associated with an increased risk of late and very late stent thrombosis12. Malappostion, coronary evaginations and uncovered struts were documented as causal mechanisms in patients suffering from stent thrombosis2,13. In CTO, the majority of stent strut malapposition is caused by late lumen enlargement due to transient impaired vasomotor function of the coronary vessel after recanalisation. This was determined with serial intravascular ultrasound (IVUS) in 40 recanalised CTO patients. Approximately 42.5% showed late-acquired malappostion accompanied by lumen enlargement in the distal vessel14. The AIR-CTO study reinforced the importance of these findings, by demonstrating reduction of in-stent restenosis and a trend towards a lower rate of definite stent thrombosis with IVUS-guided stent optimisation versus angiographic guidance only15.
This study was not powered for clinical endpoints. Therefore, the clinical relevance of differences in coverage, malapposition and coronary evaginations remains unknown. On the other hand, the low incidence of stent thrombosis in the main trial was reassuring. We have to await longer-term follow-up to assess potential late adverse events, especially after cessation of dual antiplatelet therapy.
Limitations
We acknowledge several limitations of the current study. There was no formal power calculation for the primary and/or secondary endpoints. At the time of design of the study, no OCT data were available on CTO lesions treated with DES; therefore, analyses were carried out in an exploratory context. There was no intraprocedural IVUS or OCT guidance used for stent implantation during the index procedure. Unfortunately, time-related changes and the occurrence of post-procedural and/or late-acquired stent strut malapposition, or differences in acute or late stent recoil, could not be distinguished without serial OCT investigations at both baseline and follow-up. Furthermore, the rate of post-dilatation was low in both groups. The lesion complexity of the OCT subgroup was lower than in the population of the main trial. Therefore, our findings cannot be extrapolated to a more complex CTO cohort treated with a fully embraced state-of-the-art hybrid approach.
Conclusions
The optical coherence tomography findings of this substudy demonstrated improved strut coverage with ultra-thin strut SES with bioresorbable polymer compared to thin-strut EES with durable polymer in CTO. On the other hand, SES showed a higher rate of stent strut malappositon and coronary evaginations. The clinical relevance of these findings remains to be demonstrated.
| Impact on daily practice Novel-generation drug-eluting stents with ultra-thin struts and biodegradable polymers have been developed to improve coronary healing and reduce stent thrombosis. The current study assessed stent strut coverage and apposition of the novel ultra-thin strut SES with biodegradable polymer against thin-strut EES with permanent polymer in chronic total occlusion, as a surrogate endpoint for future adverse events. The main findings were improved coverage with SES and, on the other hand, a higher rate of malapposition and coronary evaginations. We have to await long-term follow-up to assess the true risk of these findings. |
Funding
The study was supported by unrestricted research grants from Biotronik, Berlin, Germany, and Abbott Vascular, Santa Clara, CA, USA.
Conflict of interest statement
The authors have no conflicts of interest to declare.

