Abstract
Optical coherence tomography is a recently introduced intracoronary imaging modality with higher spatial resolution than intravascular ultrasound. For this reason, it is increasingly applied to investigate the characteristics of vulnerable atheromatous plaques and the result of stent implantation, struts coverage and restenosis, as well as in procedural decision-making. OCT is also useful in the field of secondary revascularisation, particularly in assessing implanted stents, evaluating relevant technologies like biodegradable stents, and in studying surgical grafts. In this article we perform a review of current developments and evidence in this area.
Introduction
Optical Coherence Tomography (OCT) is a light-based intracoronary imaging modality. Its ability to provide high resolution (in the range of 15 micron) images has revealed new aspects of the acute and long-term effects of coronary interventions not previously recognised. The technical development leading to the simplification of the acquisition procedure has extended its use and OCT systems are now available at many catheterisation laboratories worldwide. The present article aims to illustrate the clinical potential of OCT for the evaluation of patients with previous coronary interventions.
OCT for the assessment of implanted stents
OCT can be a valuable tool for the evaluation of the long-term impact of stent implantation on the coronary artery. This technique has unique capabilities for the detailed assessment of strut apposition and tissue coverage1. OCT can increase our understanding of the mechanisms implicated in the pathogenesis of in-stent restenosis. Furthermore, it can provide unique information for the evaluation of new generation bioabsorbable stents.
Stent apposition (Figure 1)
Incomplete stent apposition (ISA), also referred to as “malapposition”, is defined as a separation of a stent strut from the vessel wall. The separation distance is measured from the endoluminal side of the stent strut to the leading edge of the vessel wall. As drug-eluting stents (DES) are typically composed of a metal frame that is visible by OCT as a highly reflective structure and covered by a polymer containing the drug that is not visible, the separation distance must be larger than the strut thickness (metal plus polymer) for each specific stent type.
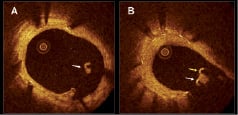
Figure 1. Incomplete stent apposition in optical coherence tomography. A: example of a malapposed strut covered by tissue (white arrow). B: example of malapposed struts in relation with a side-branch. One of the struts is covered by a thin layer of tissue (white arrow) while in the other no tissue coverage is visible (yellow arrow).
The clinical significance and long-term influence of ISA is poorly understood. In principal, ISA can (i) persist in this configuration (persistent ISA); or (ii) gain complete vessel wall contact over time (resolved ISA). Furthermore, ISA can be (iii) acquired over time whereas struts showed complete attachment to the vessel wall immediately after stent implantation, however become clearly separated from the vessel wall over time (late acquired ISA). The clinical impact and mechanism of late acquired ISA is poorly understood as well. Because OCT has a much higher accuracy than IVUS to assess strut apposition (even when the struts are covered with tissue)2, it may provide new insights into its pathogenesis. Chronic stent recoil or dissolution of a thrombus jailed between the stent and vessel wall in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction, have been proposed as possible causes of late ISA. Recently, we have demonstrated that patients who underwent DES implantation for ST elevation myocardial infarction (STEMI) showed a higher incidence of incomplete strut apposition than patients with stable or unstable angina3. Different follow-up studies have characterised the degree of stent apposition for different stent types. Several authors have reported on a higher frequency of malapposed struts by OCT in sirolimus-eluting stent (SES) than in bare metal stents (BMS)4,5. Another study evaluating SES at six months follow-up showed that malapposed struts were more often located in areas of overlapping stents6. More specifically, up to 40% of struts in overlapping segments of DES are malapposed despite high pressure dilatation7. The authors hypothesised that the presence of incomplete stent apposition could be associated with delayed endothelialisation and increased risk of stent thrombosis in overlapping regions7. OCT has also demonstrated malapposition in severely calcified lesions, even after high pressure balloon dilatation and rotational atherectomy8. Strut thickness and cell design can have an impact on incomplete stent apposition. An OCT study observed incomplete stent apposition more frequently in stents with closed design cells and thicker struts9. IVUS data have suggested a possible relation between incomplete DES apposition and subsequent stent thrombosis10. However, the clinical impact of incomplete stent strut apposition as detected by OCT is not well established. In fact, OCT is able to visualise incomplete strut apposition with a much higher sensitivity and specificity than IVUS, but this observation has not been associated with an increase in clinical adverse events5,9. As a corollary, not all patients that experience DES thrombosis show strut malapposition11,12.
Struts coverage following DES implantation (Figure 2)
Endothelial strut coverage has been identified as the most powerful histological predictor of stent thrombosis13. It is unclear, however, to what extent these post mortem findings in a very select population can be translated into the clinical arena given that it is difficult to assess strut endothelialisation in vivo. DES typically inhibit neointimal proliferation to such an extent that it may not be detectable by IVUS. On the other hand, OCT can visualise and quantify with high sensitivity and reproducibility very thin layers of tissue covering stent struts14,15. Clinical OCT studies are currently shedding light on the complex vascular healing process after stenting.
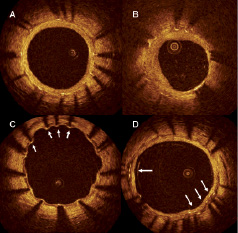
Figure 2. Tissue coverage at follow up in drug eluting stents. A: Symmetric tissue coverage: all the struts are covered by a layer of tissue of similar thickness. B: asymmetric tissue coverage: some struts are covered by a thick layer of tissue while in others (white arrows) only a small layer of tissue coverage is visible. C: the majority of struts are covered by a thin layer of tissue while some (white arrows) do not show visible tissue coverage. D: overlap region. All the struts are covered but some of them (white arrows) are surrounded by a heterogeneous low backscattering material.
STRUT COVERAGE IN BMS AND DES
Recently, several in vivo OCT studies evaluating strut coverage in DES and BMS at different time intervals have been published. Xie et al compared tissue coverage at three months follow-up in 16 and 24 patients who underwent implantation with BMS and SES, respectively. The neointimal thickness per strut was higher in the BMS than in the DES group and the frequency of struts with no visible coverage by OCT was higher in the SES group. However, no significant difference in the incidence of in-stent thrombus was observed in this small cohort5. Another study comparing BMS at short- and long-term follow-up with SES showed similar results4.
CHANGES IN DES STRUT COVERAGE OVER TIME
Matsumoto et al compared 6-month IVUS and OCT findings in 34 patients implanted with a total of 57 SES. In this study, tissue coverage was appreciated in 36% of the struts on IVUS (median tissue thickness 52.5 µm, 25th and 75th percentiles 28 and 147.6 µm respectively). In contrast, OCT identified 89% of the struts with tissue coverage, but only 16% of the stents had complete stent coverage6. In another OCT study, only 18% (8/42) of SES implants showed complete tissue coverage by six months. Furthermore, tissue coverage thickness increased from 6 to 12 months (from 42±28 µm to 88±32 µm) but only 41% of the stents had complete tissue coverage by 12 months16. Other investigators using OCT have demonstrated that 81% of patients have struts with incomplete tissue coverage two years after implantation with SES. The presence of struts without visible tissue coverage was more frequent at side branches and at overlapping segments. Findings suggestive of thrombus were observed in three patients but there were no cases of clinical stent thrombosis17.
RELATION BETWEEN DES COVERAGE AND CLINICAL PRESENTATION
Clinical presentation has been associated to the presence of struts without visible tissue coverage by OCT. Kubo et al reported that patients with unstable angina presented more frequently with incomplete stent apposition and “uncovered” struts than patients with stable angina18. Our group has shown that struts without visible tissue coverage by OCT are more frequent in patients receiving DES during primary PCI for STEMI than in patients treated with DES for stable or unstable angina3. A possible explanation for this observation could lie in the differences in the underlying plaque. Pathological studies have demonstrated a higher frequency of uncovered struts in stents implanted over lipid-rich high-risk plaques as compared with stents implanted over plaques with stable morphology19. Investigations such as the recently presented HORIZONS-OCT substudy comparing paclitaxel-eluting stents vs BMS implanted for STEMI will provide more information about the coverage of different stent types in specific clinical scenarios20.
COMPARISON OF TISSUE COVERAGE IN DIFFERENT DES
Initial results of several studies comparing tissue coverage in different DES have been reported in the last months. The OCT substudy of the LEADERS compared the differences in tissue coverage at nine months between a durable polymer sirolimus-eluting stent (Cypher Select, Cordis, Johnson&Johnson, Miami, FL, USA) and a erodible polymer biolimus A9-eluting stent (Biomatrix III, Biosensors, Morges, Switzerland). When applying a threshold of 95% of struts covered by OCT, the bioabsorbable polymer stent achieved a higher rate of coverage.21 The ODESSA (OCT for DES Safety) study compared tissue coverage at overlapping sites in BMS and different DES types (SES, paclitaxel-eluting and zotarolimus-eluting stents). The authors reported a trend towards a higher frequency of uncovered and malapposed struts at overlapping sites and differences in the number of uncovered struts and the amount of tissue coverage between different DES types22.
The effect of implanting several stent strut layers (e.g., techniques used for bifurcation treatment) or overlapping stents with different eluting drugs on strut coverage may also be studied with OCT23.
Interpretation of strut coverage by OCT should be done with caution. While OCT has been proven to reliably visualise very thin tissue layers covering stent struts, its resolution is limited to 15 micron and thus, a single cell layer can not be visualised with OCT. Second, the presence of tissue coverage should not be considered synonymous with recovery of normal endothelial function. Furthermore, it is important to realise that several OCT studies have reported the presence of uncovered struts at follow-up, but these findings were not associated with clinical adverse events17,18. Specially designed studies, including long-term follow-up, are warranted to better understand the clinical significance of incomplete strut coverage observed by OCT.
Assessment of restenosis
OCT can be a very valuable tool for the assessment of restenosis through its ability to image the neointima and identify possible causes contributing to restenosis. Animal and human data suggest that restenosis is a response to vessel injury during stent implantation. High-pressure stenting techniques have demonstrated their usefulness for stent optimisation but they can increase periprocedural vessel damage. OCT is a highly sensitive imaging modality that can detect acute complications after stent implantation (e.g. edge dissection, intrastent dissections and tissue prolapse between the stent struts)2. The clinical relevance of these findings on OCT, and their relation to restenosis, are not well established. Serial OCT studies could help to understand a possible link between periprocedural vessel trauma and future restenosis in DES.
Potential mechanisms implicated in the pathogenesis of restenosis can be readily identified with OCT; these may include gaps between stents, incomplete lesion coverage or stent under-expansion24. Stent fracture with subsequent defects in local drug delivery has been associated with restenosis after DES implantation, especially with SES. Stent fractures are identified on OCT by the lack of circumferential struts as well as distortion of stent and lumen geometry25.
Preclinical and IVUS studies have confirmed that the non-uniform distribution of stent struts can affect drug delivery and thus have an influence on restenosis. Strut distribution can be studied in vivo with OCT, where preliminary experiences in phantom models demonstrated significant differences between different DES types26. The effect of different devices used to treat restenosis (such as cutting balloon or scoring balloon) can also be assessed with OCT27,28.
Furthermore, OCT can provide further insight into the association between restenosis and other entities such as stent thrombosis11.
Due to its high resolution, OCT is able to provide detailed characteristics of restenotic lesions that were not previously appreciated by IVUS. It has been previously noted that the presence of a high reflective endoluminal layer and a low reflective abluminal layer around the struts may represent a layered structure of the neointima indicating the presence of different tissue components in the restenotic tissue29,30 (Figure 3).
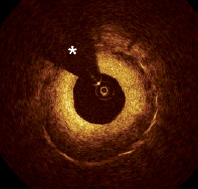
Figure 3. Stent restenosis. Stent restenosis 4 months after implantation of a drug eluting stent. Note the layered appearance of the restenotic tissue with a inner high backscattering layer and an external low backscattering layer. * guidewire artifact.
OCT can also quantify the symmetry of tissue coverage. This has been shown to be related to clinical presentation3. Furthermore, some data suggest that OCT might be able to detect the presence of neovasculature in the neointimal tissue31. The clinical implications of these OCT findings in restenotic lesions remains unknown and will require further evaluation.
Evaluation of new generation DES
Among the new stent technologies, biodegradable stents have emerged as one of the most promising. Metallic stents remain forever in the vessel as foreign material with the potential risk of stent thrombosis. In case of the need for secondary revascularisation, metallic stenting can preclude surgical treatment. Furthermore, they do not allow proper assessment by noninvasive imaging modalities such as multislice computed tomography or magnetic resonance imaging. A fully biodegradable stent that could provide scaffolding and drug delivery until the vessel has healed and then disappear completely might potentially avoid the previously mentioned drawbacks of permanent metallic stenting. OCT has demonstrated its value for the evaluation of bioabsorbable stents through its ability to image in vivo the morphological changes of the stent and the vessel wall during the absorption process. The ABSORB trial showed the feasibility of implantation of the bioabsorbable everolimus-eluting coronary stent (BVS; Abbott Laboratories, IL, USA), composed of a poly-L-lactic acid backbone, coated with a degradable polymer /everolimus matrix. OCT was performed in a subset of patients after stent implantation, at six months and at two years follow-up. In this group of patients, OCT was able to demonstrate temporal structural changes in the bioabsorbable stent with a reduction in the number of visible struts and modifications in the appearance of the struts32,33. OCT has also been able to characterise the degradation process of the bioabsorbable magnesium-based AMS stent (Biotronik; Erlangen, Germany) in a porcine coronary model34.
OCT for the assessment of surgical coronary grafts
In the past, OCT imaging of coronary grafts has been limited by challenging vessel anatomy, the vessel size and the need of vessel occlusion. Some of those limitations, however, have been supplanted by advances in OCT technology. For instance, OCT can now be performed without the need of vessel occlusion. In the new generation systems (also called ODFI, swept source, Fourier domain) the optical probe is integrated in a short monorail catheter with better navigability properties than the current commercial systems (ImageWire™). This may facilitate imaging of vessels with complex anatomy such as coronary grafts. The increase in the scan diameter may also allow visualisation of bigger vessels. Preliminary experience, in select cases in our centre, has demonstrated that in vivo imaging of venous and arterial coronary grafts with OCT is feasible and can provide valuable information (Figure 4). IVUS studies have suggested that saphenous vein grafts (SVG) undergo an “arterialisation” process with intimal fibrous thickening, medial hypertrophy and lipid deposition that creates an echolucent zone around the vessel that mimics the arterial external elastic membrane (EEM)35. In early stage SVG, OCT shows wall thickening with a mono-layered appearance and without a visible EEM (Figure 5). In later stages, the extent and distribution of the atherosclerotic disease along the graft can be visualised with great detail. After stenting OCT can assess strut apposition and vessel injury (Figure 5). SVGs are known to have a high risk of non-reflow phenomenon after stenting. This has been related to the presence of distal embolisation during stent implantation. The presence of mobile elements inside SVG (potentially at risk of embolisation) has been described by IVUS and can be visualised with higher accuracy by OCT (Figure 6). At follow-up, OCT can provide unique information about the characteristics of stent restenosis in grafts (Figure 6).
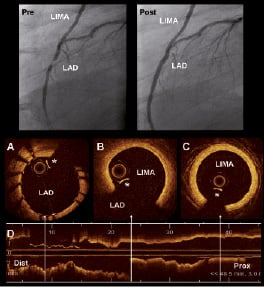
Figure 4. Optical coherence tomography (OCT) visualisation of left internal mammary artery (LIMA). The superior panel shows the angiogram of a patient with a graft of LIMA to the LAD. The left panel (Pre) shows a significant lesion on the LAD distal to the anastomosis of the LIMA. The right panel (Post) shows the result after implantation of a stent in the LAD. A: OCT cross section of the LAD with the implanted stent. B: OCT cross section on the region of the anastomosis of the LIMA to the LAD. C: OCT image of the LIMA that shows only intimal thickening without signs of significant atherosclerosis disease. D: longitudinal OCT view. The arrows indicate the location of the cross sections A, B and C. * Guidewire artifact
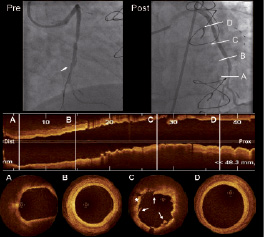
Figure 5. Optical coherence tomography (OCT) visualisation of saphenous vein graft (SVG) after stenting. Pre: angiogram showing a significant focal stenosis in a SVG to the obtuse marginal (white arrow indicates the distal protection device, FilterWire™). Post: angiographic result after treatment of the lesion with stent implantation. A to D show OCT longitudinal and cross sections images of the SVG after treatment. A: distal segment of the SVG showing low backscattering plaque with diffuse borders (suggestive of lipid content) but without significant lumen stenosis. B: SVG showing wall thickening (more pronounced at 5 o’clock). C: SVG in the stented region. The stent struts are well apposed and it can be observed the presence of tissue prolapse (*) and intrastent dissections (white arrows). D: OCT cross section of the proximal SVG segment. The vessel shows mono-layered appearance with minimal wall thickening and without signs of significant atherosclerotic disease. Dist: distal Prox: proximal.
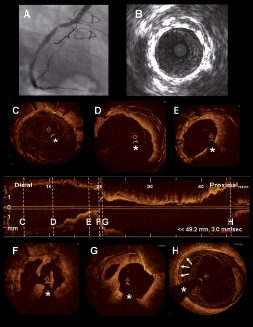
Figure 6. Saphenous vein graft restenosis imaged with optical coherence tomography. The figure shows the case of a patient treated with coronary artery bypass surgery in 1994. Saphenous vein grafts (SVG) were implanted in the LAD, diagonal, obtuse marginal and RCA. In 2003 and 2005 the patient underwent stent implantation in the SVG to the RCA for acute coronary syndrome. In 2008 the patient was referred to the catheterisation laboratory for stable angina. A: coronary angiogram showing a stent restenosis in the SVG to the RCA. B: IVUS image in the region of the restenosis showing different stent layers and severe neointimal growth. C to H show OCT longitudinal and cross sectional images of the SVG to the RCA. C: distal part of one of the stents previously implanted in the SVG. D: shows the different layers of stents previously implanted in the SVG. E: restenosis area. Multiple stent layers and severe neointimal growth are visible. F and G: restenosis area showing severe neointimal growth and irregular material protruding in the lumen. H: proximal venous graft showing a calcified plaque (white arrows) but without lumen stenosis. * Guidewire artefact.
Recent reports suggest that OCT could be useful as an intraoperative tool to select conduits for coronary artery bypass graft surgery (CABG). The outcome of CABG is related to pre-existent pathology on the harvested vessel and with vessel injury during the harvesting. Brown et al evaluated the characteristics of radial arteries and saphenous veins with OCT obtained from 35 patients scheduled for CABG. They demonstrated that OCT was able to visualise atherosclerotic lesions in the radial arteries and vessel injury related to the harvesting process (intimal trauma, thrombus)36. The same group showed that OCT is also able to evaluate and quantify the degree of spasm and vessel injury in radial arteries harvested with different methods (harmonic scalpel vs electrocautery; open vs endoscopic harvest)37,38. This ability to assess the quality of the conduits during harvesting opens new possibilities for the application of OCT in the surgical field.

