Abstract
Aims: The purpose of this single centre registry is to assess safety and feasibility of the frequency domain optical coherence tomography (FD-OCT) system during coronary interventions.
Methods and results: Ninety patients with unstable or stable coronary artery disease were included in this study. OCT imaging was performed in a first group of 40 patients (group 1), to evaluate ambiguous/intermediate lesions (24 patients in group 1 had OCT also done post-PCI, for assessment of stent deployment); and in a second group of 50 patients (group 2), to address the adequacy of stent deployment. Therefore, 74 patients underwent FD-OCT after stent implantation. A complex-lesion population was studied (B2 type lesion=72.2% and C type lesion=20.3%). The mean time of a FD-OCT pull-back (from the set up to the completion of the pull back) was 2.1 min and in all but one (99.1%) the procedure was successful. No patients experienced major complications in terms of death, myocardial infarction, emergency revascularisation, embolisation, life-threatening arrhythmia, coronary dissection, prolonged and severe vessel spasm and contrast induced nephropathy. In the ambiguous lesion group, 60% of patients were treated with PCI, whilst in the others, PCI were deferred. In total, 113 deployed stents (33,6% chromium cobalt stent, 66,4% drug eluting stent) were imaged with OCT. OCT findings led to additional interventions in 24 out of 74 patients (32%): 15 had further balloon inflations,nine had additional stent deployment whilst two had both treatments. At clinical follow-up, (4.6±3.,2 months), there were no death, acute myocardial infarctions and cases of stent thrombosis, whilst two patients underwent revascularisation for recurrence of angina.
Conclusions: The present registry shows that FD-OCT is a feasible and safe technique for guidance of coronary interventions. Randomised studies will confirm whether the use of FD-OCT will improve the clinical outcome.
Introduction
Optical coherence tomography (OCT) has emerged as a breakthrough imaging technology and promises to ameliorate clinical outcome after intervention1-9.
The new Frequency Domain Optical Coherence Tomography (FD-OCT) system can acquire images at line rates at least 10 times faster than the time-domain systems without loss of image quality and can, therefore, be used to guide coronary intervention. In fact fast pull-back are essential for imaging long segments of coronaries, fasten procedures and minimise the patient risk10,11.
The marked simplification of the FD-OCT acquisition procedures and the inherent reduction in contrast volume, as compared to the previous time domain technology, should also translate into a high procedural safety and efficacy.
The purpose of this single centre registry is to assess, for the first time, safety, feasibility and decision making impact of this new FD-OCT generation system to guide coronary interventions.
Methods
Study population
The study was conducted in compliance with the San Giovanni-Addolorata Hospital Bioethics Committee guidelines and received its approval. Informed consent was obtained for every patient.
Ninety non-consecutive patients referred for coronary angioplasty with silent ischaemia diagnosed with stress testing, stable angina or acute coronary syndrome (including acute myocardial infarction) entered the registry in the period between March and December 2009.
Presence of cardiogenic shock, renal insufficiency with basal creatinine level >2.0 mg/dL or coexistent condition associated with a limited life expectancy (i.e., advanced cancer) were exclusion criteria.
Based on the requirements of the internal FD-OCT registry, OCT images were obtained: 1) before a possible intervention to judge ambiguous or intermediate lesions at visual and quantitative coronary angiography (QCA) and decide whether to proceed with angioplasty and 2) in all cases treated with interventional procedures, as a final look, after achievement of an optimal visual angiographic result.
In all patients, clinical follow-up was scheduled at 1, 3, 6 and 9 months after discharge, with a mean value of 4.6 +3.2 months.
FD-OCT procedure
The LightLab FD-OCT system is equipped with a tuneable laser light source with sweep range of 1.250 to 1.370 nm. The optical fibre is encapsulated within a rotating torque wire built in a rapid exchange 2.6 Fr catheter. The system has been already tested in humans in Europe and Japan.
The FD-OCT imaging catheters, were delivered over a 0.014 inches guidewire through a 6 Fr or larger guiding catheters, after administration of intracoronary nitrates. For an effective clearing of blood from the imaging field, an angiographic contrast media was injected through guiding catheter adopting an automated modality12,13. Injection of 14 ml volume of contrast at a rate less than 4 mL/s was sufficient to achieve an imaging period of 2-3 sec consistently in all of the major coronary branches. At a pull-back rate of 20 mm/sec, an imaging period of 2 sec was long enough to scan 4 cm vessel segment.
FD-OCT images were calibrated adjusting the Z-offset. This critical step was done before image acquisition as to obtain accurate measurements14.
PCI procedures and adjunctive medical therapy
Percutaneous coronary intervention (PCI) was performed using standard technique via the femoral or radial approach. Patients received either drug eluting stent (DES) or bare metal stent (BMS) at the discretion of the operator. All patients were treated with aspirin 325 mg prior to PCI and loaded with clopidogrel 600 mg if not already on a maintenance dose. Dual antiplatelet therapy was recommended to all study patients for a minimum duration of six months. During PCI, patients were anticoagulated with unfractionated heparin (a bolus of 40 U/kg and additional heparin to achieve an activated clotting time of 250–300 sec). Use of platelet glycoprotein IIb/IIIa inhibitors was at the operator’s discretion.
Definitions of safety, feasibility and impact on decision making during intervention
Safety
The registry included any complications judged by operator that occurred during or within the immediate 24 hours period following FD-OCT examination, even if transient. As previously published15 the following events were categorised: death, myocardial infarction, emergency revascularisation, embolisation, life-threatening arrhythmia, coronary dissection, prolonged and severe vessel spasm and contrast induced nephropathy (CIN). CIN was defined as an increase in serum creatinine concentration of >0.5 mg/dL (>44 micromol/L) or 25% above baseline within 48 hours after contrast administration.
Feasibility
Feasibility was defined as the ability to cross lesion or stent struts with image wire and to obtain a good quality of imaged coronary segment in terms of accurate measurement of: 1) vessel luminal area with classification of the superficial plaque components; and 2) stent luminal area with good visualisation of stent struts13.
Impact of FD-OCT on decision making during intervention
Impact of FD-OCT on decision making during intervention was defined as its ability to affect the operator strategy at baseline and after intervention. This consisted in a) treating or not hazy or intermediate angiographic lesions at baseline and b) deciding at post intervention whether to proceed to additional balloon dilatation in presence of non optimal stent positioning or to perform further stent implantation to treat vessel dissections at the stent edges.
Plaque assessment
All OCT frames were evaluated on-line in the cathlab by personnel with specific expertise in OCT imaging. Atherosclerotic plaques were classified based on previously published criteria7. Ambiguous lesions on angiography were defined as lesions with haziness regardless of stenosis degree or as lesions with intermediate severity (diameter stenosis of 40 to 70% at QCA)16. The selected OCT criteria for the treatment of ambiguous lesions were: 1) presence of thrombus indicative of unstable lesion, in case of haziness at angiography7; 2) minimum luminal area (MLA) <3.5 mm2.16
Stent assessment
Stent assessment after deployment was based on the evaluation of the following parameters:
1) optimal stent expansion8,14 was defined according to the previously reported MUSIC criteria17: achievement of in-stent MLA >90% of the average reference lumen area, with a symmetric stent expansion defined by minimum lumen diameter/maximum lumen diameter >0.7; 2) strut apposition was defined based on published criteria5 and was considered significant if the distance between strut and vessel wall was greater than 200 µm and had a length of at least 600 µm (corresponding to three contiguous frames for pullback speed of 2 cm/sec); 3) tissue-prolapse, was defined as protrusion of tissue towards the lumen, and was considered significant if the distance from the stent struts to the greatest extent of protrusion was >100 µm18; edge-dissection, defined as disruption of the luminal vessel surface in the edge segments (within 5 mm proximal and distal to the stent). An FD-OCT guided strategy of further stent deployment was employed in case of stent induced vessel wall dissection extending beyond 200 µm, while further balloon dilatation was performed in the presence of non optimal stent expansion and/or significant strut malapposition or when tissue prolapse >100 µm was present.
Statistics
The distribution of continuous variables was assessed by visual estimation of frequency histogram and by means of the Shapiro Wilk test. Continuous variables were expressed as mean±standard deviation (SD) or median±interquartile range, if they fit a normal or non-Gaussian distribution, respectively. Categorical variables were expressed as percentage. Continuous variables were compared with unpaired t-test or Mann Whitney U-test and categorical variables with chi-square or Fisher’s exact test, as appropriate. A two tailed p value <0.05 was considered statistically significant.
Results
FD-OCT guided interventional procedures were planned in 90 patients.
In 40 patients OCT was performed for evaluation of ambiguous/ intermediate lesions: as 24 patients were treated with stenting, they had OCT done pre- and post-intervention, whilst the remaining 16 patients were left untreated. In a second group of 50 cases we attempted to obtain OCT images only post-intervention to address the adequacy of stent deployment. Therefore a total of 74 patients had OCT done post-stenting (Table 1).
Table 2 shows demographic, clinical and angiographic characteristics of the studied population with 37% of patients having an acute coronary syndrome (ACS). A complex-lesion population was studied; in fact 7.4% of the target lesions were type B1, 72.2% type B2 and 20.3% type C according to the ACC classification. QCA was performed in the group with basal FD-OCT assessment and measured the mean target lesion stenosis at 50.1±5.85%.
Safety and feasibility of FD-OCT
In total 114 OCT acquisitions were performed in 90 patients. In all but one (99.1%) the procedure was successful. The mean time of a FD-OCT pull-back (from the set up to the completion of the pull back) was 2.1±0.54 min. The mean amount of contrast administered to each patients, was 217±91.2 cc with an average volume of 49.4±19.0 cc specifically required for OCT assessment. No patients suffered CIN. The mean creatinine value varied from 1.17±0.12 cc pre-intervention to 1.21±0.19 cc post-intervention (p=n.s.). No major complications were recorded for image wire mechanical entrapment. In one patient, referred to our institution for unstable angina, with haziness at angiography, the image-wire negotiation led to a transient vessel spasm that was resolved with intracoronary administration of nitrates.
During FD-OCT images acquisition no ischaemic ECG changes occurred. Ventricular ectopic beats were found only in three patients during contrast infusion for blood clearance, while other major arrhythmias (ventricular tachycardia of fibrillation) were not observed.
In one case only, in which FD-OCT was performed to address two overlapped stents, the OCT probe was unable to cross the distal stent as it was stuck at the site of overlapping. In all other cases OCT was able to negotiate the lesion or the stent and images were adequately obtained despite the fact that OCT was done also in vessels with marked tortuosity and calcifications.
Impact of FD-OCT on decision making during intervention
Plaque assessment
Pre-intervention FD-OCT was performed to evaluate ambiguous/ intermediate lesions (Figure 1) at basal angiography in 40 patients; eight patients (20%) had an ACS and haziness at angiography, whilst the remaining 32 (80%) had intermediate narrowing and a stable syndrome. Of the 40 patients with ambiguous/intermediate lesions 24 (60%) were treated with PCI, whilst in the remaining 16 (40%) the coronary interventions were deferred.
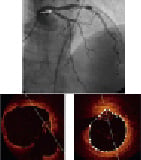
Figure 1. Upper panel: angiography shows an intermediate lesions at the left anterior descending (LAD) ostium (white arrow) and a significant lesion at mid LAD (black arrow). Lower panel: FD OCT imaging of distal left main bifurcation (left panel) and ostial LAD (right panel) show a non significant narrowing with a minimal lumen area of 7.8 mm2. PCI was done only at mid LAD.
OCT findings among the 24 patients undergoing PCI were a minimal lumen area less than 3.5 mm2 in 19 (79%) patients and presence of thrombus with signs of plaque ulceration indicative of unstable plaque in the remaining five (21%) patients.
Stent assessment
Post-intervention FD-OCT was performed in 74 patients to verify the adequacy of stent deployment and absence of vessel injury at reference sites. In total, 113 deployed stents were imaged with OCT. Thirty-eight (33.6%) were chromium cobaltum stents, whilst 75 (66.4%) were drug eluting (Cypher, [Cordis, Johnson & Johnson, Warren, NJ, USA] 36.0%; Taxus, [Boston Scientific, Natick, MA, USA] 34.7%; Endeavor, [Medtronic, Minneapolis, MN, USA] 26.6%; Xience, [Abbott Vascular, Redwood City, CA, USA] 2.7%). Mean nominal stent diameter was 2.87±0.37 whilst mean length was 18.08±6.42. Stents were deployed at an inflation pressure of 17±8 atm.
OCT findings led to additional interventions in 24 out of 74 patients (32%): 15 had further balloon inflations,nine had additional stent deployment whilst two had both treatments.
The additional balloon inflations were done: to correct a significant malapposition (one case) (Figure 2), to treat a significant tissue prolapse (four cases), to treat under expansion (three cases) and for the presence of multiple criteria (seven cases). In all the nine cases who received an additional stent, OCT showed a significant dissection at stent edges (Figure 3). Lastly in two cases both further balloon expansion and stent deployment were accomplished due to presence of dissection and under expansion in one case and dissection and malapposition in the second one.
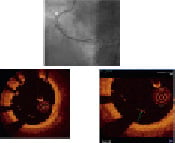
Figure 2. Assessment with FD-OCT after positioning of a chromium cobaltum stent in the right coronary artery. Angiography (upper panel) shows a well deployed stent in the proximal RCA (white arrow); FD-OCT imaging (left bottom) reveals, between 4 and 9 o’clock, a marked stent struts malapposition, that was found in seven contiguous frames. Magnification (right bottom) of the same FD-OCT image, shows the measurement of stent struts malapposition (max 670 µm). Based on this OCT findings, a further dilatation with an oversized balloon was done.
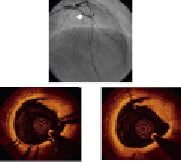
Figure 3. Upper panel: angiography shows good result after deployment of a drug eluting stent on the mid LAD (white arrow); the black arrow shows the distal edge of the stent. Lower panel: FD OCT assessment after stent deployment. OCT reveals a significant rime of dissection in the distal edge of the stent (left) and one frame distally (right), not visible angiographically.
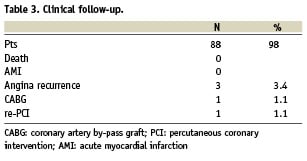
Clinical follow-up (Table 3) was obtained in 88 patients with a mean duration of 4.6±3.2 months. The minimal duration of follow-up was 1 month. There were no deaths, acute myocardial infarctions and cases of certain, probable or possible stent thrombosis (Academic Research Consortium, ARC, definitions)19. Three patients had a new occurrence of chest pain and two of them underwent revascularisation. Due to occurrence of restenosis, coronary artery bypass was done in one case and re-PCI in the other; in this latter re-PCI was done on the vessel not imaged by FD-OCT at baseline. All patients in which coronary intervention was deferred, based on OCT findings, did not experience coronary events and were symptoms free.
Discussion
OCT feasibility and safety
The present paper shows that FD-OCT is a safe and feasible technique. FD-OCT ease of use is due to the fact that it simply requires a non-occlusive modality of image acquisition, that had been developed for the previous time domain OCT system, with contrast media being infused through the guiding catheter as to displace red blood cells9,10. There are few data on the safety of FD- OCT20 as the vast majority of information available in the literature refer to time domain OCT. The largest study included 468 patients from six European centres and showed low rate of ventricular fibrillation (1.1%), air embolism (0.6%) and vessel dissection (0.2%)15.
To our knowledge this manuscript provides the first available data on the feasibility and impact on decision making of frequency domain technology. The marked simplification of the acquisition procedures and the inherent reduction in the required contrast volume, has offset the procedural complication rate, by completely eliminating major arrhythmias, the most frequent complication of time domain OCT. Furthermore FD-OCT probe is miniaturised as compared to intravascular ultrasound (IVUS) technology, progressing OCT into a feasible technique. Despite the fact that in many patients from our registry, OCT probes had to negotiate complex lesions in calcific and tortuous vessels, the procedure was highly successful (99% of cases). Lastly, the fact that FD-OCT is very fast, with a 50 mm pull-back that can be obtained in less than five seconds, makes the technology very attractive for repeated fine tuning of stented artery9.
Assessment of ambiguous lesions and deferral of interventions
Sub-optimal angiographic visualisation often occurs in the every-day practice. It may happen in presence of intermediate lesions of uncertain severity, very short lesions, pre- or post-aneurismal lesions, ostial or left main stenoses, disease at branching sites, sites with focal spasm, or angiographically hazy lesions. In these circumstances intracoronary physiologic measurements including fractional flow reserve proved to be useful to address intermediate coronary stenoses in patients with anginal symptoms21 and determine whether an intervention is warranted. Morphological techniques such as IVUS and FD OCT can also be used to discriminate significant lesions from mild ones and the latter, due to its excellent delineation of the lumen-wall interface and optimal assessment of the plaque superficial composition can identify abrupt plaque ulceration and thrombotic formations that may be missed by IVUS7,13,22.
Our preliminary clinical follow-up data are very promising; they show that the use of an OCT guided strategy to address ambiguous lesions may avoid a number of unnecessary interventional procedures. However such a strategy has to be further proved by perspectives studies.
Post procedural assessment
The use of an IVUS guided approach to optimise DES is a reasonable approach to reduce the thrombosis rate23,24. Roy et al25 recently compared 884 IVUS-guided intracoronary DES implantations with the outcomes of a propensity-score matched population having angiographic guidance alone. The rate of definite stent thrombosis at 12 months, that was the primary endpoint of the study, was significantly lower in the IVUS guided group and there was a trend in favour of the IVUS group in target lesion revascularisation.
The use of OCT instead of IVUS for guidance of interventional procedures follows the concept that luminal reduction per se, more than large residual plaques, can cause flow impairment. Using this philosophy OCT, due to its ability to address reduction of lumen areas in the stented segments or at stent margins (caused by flow limiting dissection, lesion prolapse or large plaque burden), can provide interventionalists with worthwhile information capable of affecting the clinical outcome. Furthermore, OCT ability to identify intra-stent residual thrombosis is likely to affect patient follow-up. Preliminary clinical data provided by our registry on OCT guidance are encouraging. Interestingly OCT findings led to additional interventions in 32% of patients and no acute and sub-acute thrombosis of treated vessels were reported at 1 month follow-up despite the fact that 37% of patients had an ACS.
The fact that the study is non randomised does not enable definitive conclusions about OCT ability to improve the clinical outcome as compared to angiography. Further data are needed to corroborate this concept.
Selected criteria for optimal stent positioning
We adopted a final look OCT strategy of stent positioning pursuing the goal to correct stent under expansion. OCT images were acquired after achievement of an optimal angiographic result based on the concept that stent under expansion, a well known parameter related to stent thrombosis, is a rather common finding, particularly in the presence of calcified vessels. Stent minimal lumen area was measured in all cases in the attempt to achieve the most used MUSIC criteria for optimal stent expansion, which are based on the luminal stent assessment and the comparison between minimal stent lumen areas with reference ones17.
Malapposition can be misdiagnosed by IVUS and is easily visualised by OCT. This feature was one of the adopted criteria of non optimal stent positioning in spite of its uncertain clinical significance. It was felt that a complete apposition of equi-spaced stent struts is an important requisite to reduce thrombogenicity26,27.
OCT is also capable of detecting even the smallest amount of thrombus depositions on stents struts. Intra-stent thrombosis is a rather a common finding, in patients with ACS, after stenting of the culprit lesions; in the present registry 36.3% of the patients had a significant in-stent thrombus, requiring further intervention28. As for malapposition, the clinical significance of in-stent thrombus deposits is still unknown but it is reasonable to think of this feature as a possible cause for acute and sub-acute stent thrombosis, particularly in the pre-thrombotic milieu of the ACS. Obviously larger prospective OCT data are needed to corroborate all these concepts.
Limitations
The non randomised design of the study, the lack of a control group and the non consecutive selection of patients undergoing OCT guided intervention, are major limitations of the study.
Another limitation of OCT resides in its inability to measure plaque burden whose thickness exceeds 1.3-1.5 mm. This does not allow an accurate assessment of vessel architecture and the selection of stent size and diameter. In an OCT guided approach these information may be obtained by angiography, confining OCT assessment to a post-intervention final look. Alternatively, OCT will be used to obtain a pre-intervention luminal assessment. Future studies will clarify whether this major limitation of OCT will be offset by the extraordinary definition of the stent surface, provided by the technique.
OCT is a novel technique and, unlike IVUS, there is a few data on the ability of OCT to measure stent lumen areas and identify stent under-expansion. However, despite the lack of standardised OCT criteria to identify the appropriateness of stent implantation, previous data showed an excellent correlation between IVUS and OCT measurements in stent lumen area29. Therefore it is likely that IVUS luminal criteria for optimal stent measurements can be translated to OCT.
A technical draw-back of the technique is that plaques located at the very ostium of the left or right coronary arteries cannot be accurately imaged by OCT; in fact, at the current stage of technology, neither of the two OCT acquisition techniques (occlusive or non occlusive) appears to be suitable for aorto-ostial assessment7.
Conclusions
The present registry shows that an approach to coronary interventions based on the use of FD-OCT is safe and feasible. It can be used to avoid unnecessary interventional procedures and may ameliorate stent positioning. Randomised studies will confirm whether these features can improve the clinical outcome.

