Cardiovascular outcomes following percutaneous coronary intervention (PCI) steadily improve due to progress of coronary stent technology1,2, antithrombotic therapy3,4, and the use of novel diagnostic approaches5. As angiography provides only limited information, it is tempting to employ intracoronary imaging to assess the post-procedural results with the intention to further optimise outcomes. Although intravascular ultrasound (IVUS) provides more detailed information than angiography alone, its uptake has been modest to guide PCI6,7. Meanwhile, a new and more promising technology –optical coherence tomography (OCT)– has become available. By utilising light instead of sound, it provides histology-like images of stents and the adjacent vessel wall. Various post-procedural OCT findings may appear dramatic and concerning at first sight, even if angiographically invisible (e.g., dissection), and may trigger additional or even unnecessary interventions to mitigate potential adverse outcomes8. The relatively high incidence of angiographically silent abnormal OCT findings indicates that the use of intracoronary imaging tools frequently requires difficult decisions regarding the management of imperfect results. The unrivalled high resolution of OCT combined with its ease of use has raised hopes that implantation of stents in atherosclerotic coronary arteries can be further perfected. Despite the widespread use of OCT, clinical trials testing the ability of OCT to guide PCI and to assess its impact on cardiovascular outcomes have not been performed so far. It is against this background that Prati and colleagues took the initiative and proposed a pragmatic scheme to guide PCI with the use of OCT, and proved the feasibility of this concept in a small patient cohort as early as 20109. The same pioneering group of investigators presents in this issue of EuroIntervention a substantial extension of the initial patient cohort and compares outcomes with a control group10. The study included a total of 670 patients: 335 patients undergoing OCT-guided PCI, and 335 control patients treated with angiographic guidance alone. Whenever OCT showed evidence of stent malapposition, dissection or underexpansion, a therapeutic corrective measure was recommended consisting of high-pressure post-dilatation or additional stent implantation. Following adjustment for differences in baseline characteristics between the groups, the investigators observed a reduction in the primary endpoint of cardiac death or myocardial infarction (MI), suggesting a substantial benefit of OCT- as compared to angiography-guided PCI. Moreover, the authors present reassuring data on the safety and feasibility of OCT-guided PCI.
Interpretation of the results in the context of the study design
Does the data provide convincing evidence that the implementation of OCT-guided PCI will improve cardiovascular outcomes? The present study raises several important points, which require careful consideration:
First, since the study was not randomised, the use of observational data is subject to bias owing to measured and unmeasured confounding. Notably, the authors employed a case-control study design in which the sole selection criterion for the control group was the prerequisite of having undergone a PCI within 30 days of the study-matched OCT-guided PCI procedure. This confounding becomes obvious when inspecting the baseline characteristics as they differ substantially, for example, with regard to the number of patients presenting with acute ST-elevation myocardial infarction or use of bare metal stents. Accordingly, selection of the control group did not result in an adequate balance of baseline characteristics between the two groups, as in case-control studies confounding is not automatically controlled by matching11. Of note, case-control studies are well suited for studying rare diseases such as stent thrombosis. In this specific study, however, two interventions were compared and, therefore, the use of propensity matching to select the control patients would potentially have resulted in more comparable groups12. Instead, authors employed propensity scores for the purpose of adjustment rather than for selection of controls.
Are the clinical outcomes of the control group representative of patients in routine daily clinical practice? Figure 1 presents cardiac event rates obtained from recent large-scale all-comers and primary PCI trials, in which stent implantation was performed on the basis of angiographic guidance. Large differences in terms of absolute event rates become apparent with high rates of cardiac death in the angiography-guided control group, which further substantiates the notion of selection bias in this pilot study. In order to appreciate the selection process better, outcomes of the overall population treated at the three participating centres would have been of interest.
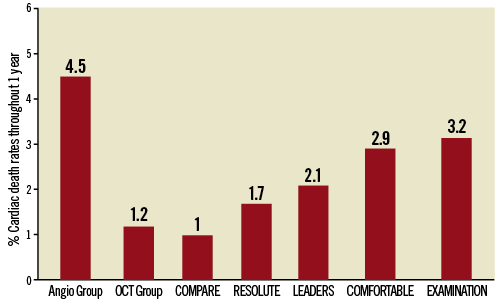
Figure 1. Cardiac death rates in perspective. Cardiac death rates at one-year follow-up as assessed in the study of Prati et al (angiography-guided and OCT-guided) are shown and compared to cardiac death rates observed in large-scale stent trials including all-comers patients COMPARE (everolimus-eluting stent); RESOLUTE (everolimus-eluting stent); LEADERS (biolimus-eluting stent), and STEMI patients COMFORTABLE (biolimus-eluting stent) and EXAMINATION (everolimus-eluting stent).
Causality between OCT intervention and improved outcomes
Is there a potential mechanistic explanation for the observed differences between OCT- and angiography-guided PCI? The study protocol mandated the treatment of OCT-detected stent underexpansion, malapposition and dissections. The presence of these features has previously been associated with an increased risk of target lesion revascularisation as well as stent thrombosis13-16. However, hazard ratios of both revascularisation and stent thrombosis throughout one year were close to one, and confidence intervals were wide, suggesting that there was no clinically overt difference in these particular outcomes, which renders a mechanistic explanation of the presented results elusive. Regarding the observed differences in MI, a distinction between target-vessel and any MI, as well as periprocedural versus spontaneous MI, would have been helpful to elucidate further the potential impact of OCT guidance on this outcome measure.
As implied by the results, the differences between groups are driven by the elimination of potentially “adverse features” such as edge dissections, strut malapposition and thrombus, which were more frequent than the presence of reference lumen narrowing and stent underexpansion (14.2%; 29.7%; 22%; vs. 2.8% and 11.4%, respectively). The proposed intriguing relationship raises a few questions:
The most important relates to the challenge of defining relevant cut-off values for OCT-detected angiographically silent adverse features justifying intervention. These were either selected arbitrarily or based on thresholds defined by IVUS in the present study, although only limited data on the natural history of OCT-defined adverse features exist to date. With respect to edge dissections, the protocol mandated additional stent implantation when the flap width exceeded 200 µm, whereas extension along the length or in the depth of the vessel was not considered. Recent studies of angiographically silent edge dissections, however, have reported flap widths measuring on average 0.67-1.20 mm which, left untreated, did not result in adverse events during short- or long-term follow-up17-19. Two of these studies included a follow-up assessment of 22 and 12 edge dissections demonstrating that 90% and 100% of these were fully healed by one year (Figure 2A) with a median of 188 days, respectively. The two cases exhibiting persistent dissection at one year had the longest flaps (2.42 mm vs. 2.81 mm) in the respective cohort at baseline19. Although there is not yet sufficient data to indicate which angiographically silent edge dissections will lead to events, and thus require additional intervention, it is thought-provoking that longitudinal extensions up to 8 mm in previous IVUS studies and circumferential extensions up to 3 mm by OCT have been associated with an uneventful long-term clinical course20. In view of the above, it would have been interesting to reveal the extent of the edge dissections in the present study. The fact that none was angiographically visible and that edge dissections previously associated with stent thrombosis either exhibited reduced TIMI flow or presented as angiographic type B-D dissections14 calls into question whether treatment of angiographically silent dissections does translate into improved outcomes.
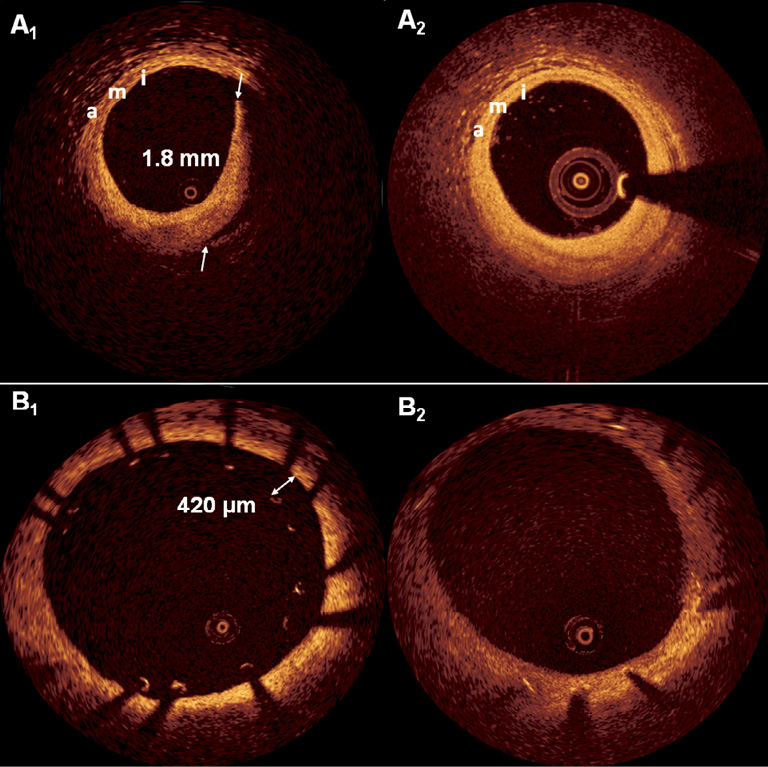
Figure 2. Examples of healed edge dissection and malapposition. Panel A1 shows a large flap with a width of 1.8 mm which underwent complete healing as visualised at one year (A2) following implantation of a zotarolimus-eluting stent. The intima (i), media (m) and adventitia (a) are indicated and confirm a good matching between baseline and follow-up. In panel B1 we see a cross-section where several struts are malapposed, with strut-lumen distances up to 420 µm. At one year following implantation of this zotarolimus-eluting stent (B2), all struts appear well apposed and covered by regular tissue, suggestive of a neointima.
As it relates to stent malapposition, operators performed additional dilatation whenever the strut-lumen distance exceeded 200 µm –a finding present in 29.7% of patients. Previous serial OCT data strongly suggest that the frequency (binary) of malapposition spontaneously decreases from baseline to follow-up21,22 through the filling of the stent-lumen gap with neointima – seen in these studies without any events at mid-term follow-up. Gutierrez-Chico et al investigated in more detail the relationship between various cut-offs for malapposition and whether or not it persisted through 6-13 months follow-up. They found that strut-lumen distances <270 µm resolved in 100% of cases and those <400 µm in 93% of cases –without any occurrence of stent thrombosis23. Considering these data, the recommendation for treatment at the present cut-off proposed by the authors should therefore have been more conservative, and a less stringent criterion may have sufficed.
OCT-guided PCI: looking ahead
With the above in mind, we can conclude that the investigation of the utility of a novel technology like OCT to guide PCI is a challenging task. Prati and colleagues should be commended for their original work, which provides reassuring evidence that OCT is a feasible and safe procedure in most patients undergoing PCI in various clinical conditions; that OCT pullbacks can be readily interpreted by the interventionalists; and that a pre-specified protocol to guide the procedure can be implemented in the setting of a multicentre study.
For the further refinement of PCI, it is crucial to understand the natural history and clinical impact of various OCT-defined abnormalities. Furthermore, it will be important to validate the effect of the additional intervention by control-imaging in order to find the balance between potential benefits and risks with additional intervention. In this regard, it is important to bear in mind that additional stent implantation to treat dissections may result in further dissections, and that the presence of overlapping stents carries the risk of adverse outcome during long-term follow-up24. Moreover, recent results indicate that further dilatation of malapposed segments may not satisfactorily correct the incomplete apposition, particularly in calcified lesions and at the take-off of side branches25. In the setting of acute coronary syndromes, the peri-stent area may be composed of plaques with large lipid cores, or thrombus of significant size, which with further dilatation may embolise distally and result in no reflow.
Considering important advances in the near future such as immediate three-dimensional reconstruction and automatic stent strut detection, the prospect of OCT becoming an indispensable imaging tool in challenging clinical situations is very promising. Indeed, for all these reasons, this readily usable imaging tool with unprecedented resolution will prevail to improve further the outcomes of patients undergoing PCI.
Conflict of interest statement
The authors have no conflicts of interest to declare.

