Intravascular optical coherence tomography (OCT) is a valuable imaging tool for guiding percutaneous coronary intervention (PCI). Previous OCT studies have demonstrated that stent abnormalities such as underexpansion, dissection, malapposition, and tissue protrusions are associated with PCI outcomes1. However, the quantitative OCT criteria of those stent abnormalities for the requirement of additional procedures have not yet been clarified. Interventional cardiologists have determined PCI endpoints based on their own experience or local rules in OCT-guided PCI. To standardise OCT-guided PCI, it is necessary to develop quantitative OCT criteria for stent optimisation.
In the current issue of EuroIntervention, Prati et al2 report the long-term (7.5 years) clinical impact of quantitative OCT criteria of suboptimal stent implantation using data from a retrospective multicentre PCI registry (named the CLI-OPCI project). Suboptimal stent implantation was defined as the presence of at least one of the following OCT findings: in-stent minimum lumen area <4.5 mm2; reference lumen narrowing with lumen area <4.5 mm2; stent edge dissection with a width ≥200 μm. These OCT findings were associated with an increased risk of cardiac death, as well as acute/subacute stent thrombosis, in-stent restenosis, or repeat revascularisation.
OCT-guided PCI is typically performed according to a step-by-step OCT workflow that guides treatment decisions pre- and post-PCI. Pre-PCI OCT is used to determine treatment strategies for PCI. The lesion morphology, such as severe calcification, should be evaluated to determine the need for aggressive lesion preparation prior to stenting. The proximal and distal reference sites should be properly determined to estimate the stent length. The reference vessel/lumen size should be accurately measured to determine the stent diameter. Post-PCI OCT is used for stent optimisation (Figure 1). Stent expansion should be maximised, malappositions should be minimised, and major stent edge dissection should be corrected. The OCT software for automated measurement and angio-coregistration supports guidance of PCI by preventing longitudinal or axial geometric mismatches3. The OCT guidance provides significant changes in PCI procedures compared to angiography guidance alone.
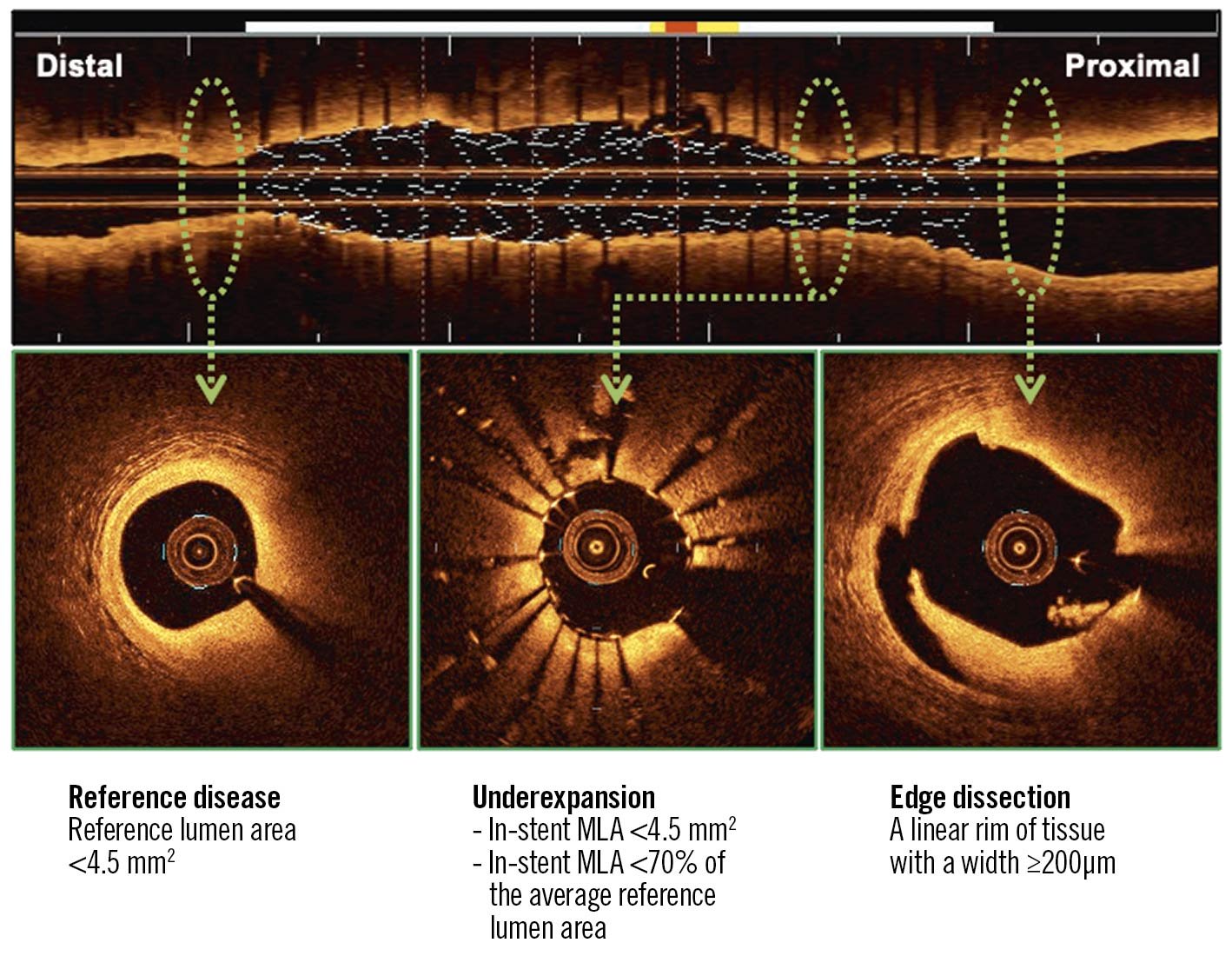
Figure 1. Quantitative OCT metrics of suboptimal stent implantation. Stent underexpansion, reference disease, and stent edge dissection are associated with worse long-term PCI outcomes. MLA: minimum lumen area; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Stent underexpansion is a significant prognostic factor. A small absolute value of the minimum stent area (MSA) is associated with an increased risk of stent failure. In non-left main coronary arteries, the optimal MSA measured by OCT is reported to be >3.5->5.0 mm21. Since the MSA depends on the vessel size, the relative expansion might be a more practical predictor. The relative expansion of >70% shown in the current study is an achievable goal in routine clinical practice. Previous studies have used a relative expansion of >80% or >90% as criteria for optimal stent implantation with OCT guidance1. However, it was not easy to meet these criteria. Moreover, in the current study, these criteria were not associated with long-term outcomes.
Untreated reference segment disease can lead to stent edge restenosis. The reference segments are rarely normal. Especially in diffuse disease, it is difficult to determine plaque-free reference segments for stent landing. In OCT-guided PCI, the regions with a lipid arc >180˚ are known to be unsuitable for reference segments1. In addition, the current study suggests that the regions with a lumen narrowing <4.5 mm2 should be excluded for reference segments (especially for the distal reference segment).
Stent edge dissection is a predictor of stent thrombosis. OCT helps determine the presence or absence of stent edge dissection when angiography shows obscure findings (e.g., haziness, filling defect, or lumen narrowing). The OCT imaging procedure with contrast injection results in a slight but significant increase in intracoronary pressure (9±2 mmHg in systole)4. It is necessary to understand the risk of extension of stent edge dissection due to the OCT imaging. The severity of dissection is evaluated by measuring the depth, axial angle, and longitudinal length of the dissection. Previous studies demonstrated that involvement of media or adventitia, a dissection angle of >60˚, and a dissection length of >2 mm were predictors of target lesion failure1. In the current study, a linear rim of dissection flap with ≥200 μm was added as a predictor of a worse long-term outcome.
Several OCT studies have shown that OCT-guided PCI can lead to a better outcome than angiography-guided PCI. However, the use of OCT is not so widespread in the real world. One reason is the lack of quantitative OCT criteria for stent optimisation. The results of the current study by Prati et al provide important information for the development of quantitative OCT criteria for stent optimisation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

