
Optical coherence tomography (OCT) allows detailed examination of coronary arteries, providing a wealth of information that is complementary to angiography1. Research into the use of this relatively new technology in interventional cardiology has established areas where OCT can be useful in guiding therapy (e.g., optimisation of percutaneous coronary intervention [PCI]). However, data on the long-term clinical impact of OCT-guided PCI optimisation are not yet available, but will be determined by ongoing randomised trials2.
Several questions remain regarding the potential use of OCT for following the natural history of coronary atherosclerosis and the acute and long-term biological response to invasive and medical therapies. In this issue of EuroIntervention, three studies are reported which seek to determine 1) the utility of OCT for guiding vessel preparation of calcified lesions3, 2) the temporal response of non-culprit atherosclerotic plaque to contemporary guideline-directed medical therapy4, and 3) the use of OCT in lieu of physiological measurements to determine whether to perform or defer PCI on stable coronary artery lesions5.
The study by Fujino et al builds on previous data on the use of OCT in guiding PCI on calcified lesions6-9, suggesting a new scoring system to predict adequate stent expansion in calcified lesions.
Indeed, OCT is superior to intravascular ultrasound (IVUS) in its ability to visualise and finely delineate calcified coronary deposits8 as it allows measurement not only of the arc of calcification but also of the thickness. One of the ongoing dilemmas for interventionalists is the optimal approach for “lesion preparation” in calcified plaques - whether to use non-compliant balloon inflation only or cutting or scoring balloons, or to perform atherectomy upfront and, if so, when and why. The challenge is that fluoroscopic assessment is considered inadequate for reliable prediction of the response to balloon dilatation and thus subsequent stent expansion.
By visualising calcium boundaries (cross-sectional thickness, longitudinal length and circumferential arc) as well as the position of the calcified plaque within the three layers of the coronary artery, OCT allows comprehensive geometric and quantitative assessment of calcified lesions6-8. The proposed scoring system combines the three-dimensional measurements - calcium thickness (shown previously to impact negatively on stent expansion10), calcium length and arc, with each component weighted and scored in a binary fashion in relation to thresholds that were determined by receiver operating curves and a multivariate regression model in a test population3. It follows that the summative interaction of these components reflects the three-dimensional volume of the calcium deposits and their response to balloon inflation/stent expansion - the larger the burden, the lower the possibility of adequate stent expansion. Indeed, there was significantly lower stent expansion in target lesions with a maximum score of 4 in the validation cohort, whereas stent expansion in lesions with scores of 3 or less was generally acceptable.
Different mechanisms are expected to underlie adequate stent expansion at different combinations of the individual components of the scoring system. The study by Fujino et al does not elaborate on the mechanisms of plaque modification by balloon expansion, stratified by the calcium score, in particular on calcium fracture known to be associated with greater stent expansion6,8. While it is expected that balloon dilatation would induce fracture in a calcified sheet with a maximum calcium arc of >180° and thickness of <0.5 mm7 (case A, figure 3, calcium score 2)3, deep thick nodular calcium may not physically impede stent expansion and be displaced by the stent (case B, figure 3, score 1). While the study excluded lesions where specialty balloons or atherectomy devices (rotational, orbital or excimer laser) were used to modify the calcific plaque, and did not include patients with end-stage renal failure where distinct coronary calcification patterns are observed11, it does nonetheless represent a critical step towards advancing our understanding of plaque modification in calcified lesions. The scoring system may be used in prospective studies to investigate the association with the effects of various calcium modification modalities, to protocolise decision making in the use of obligatory adjunctive atherectomy (Figure 1) and to determine the association of calcium score with acute procedural results and longer-term clinical outcomes. These types of studies may incentivise commercial development of software that could automatically render 3D volume measures of calcium severity, streamlining the use of OCT to guide clinicians in selecting the use of an appropriate treatment strategy for severely calcified coronary lesions.
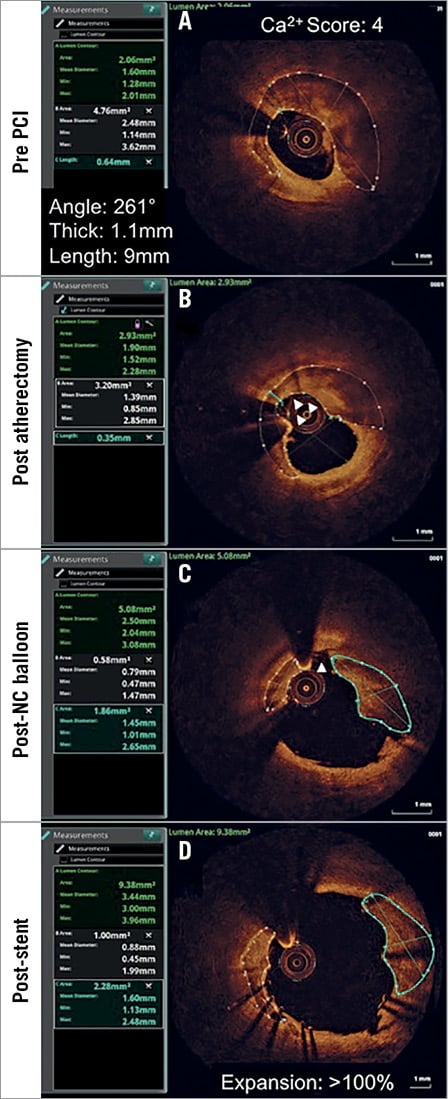
Figure 1. Calcium score-directed plaque modification. A) A middle left anterior descending severely calcified coronary stenosis, with a calcium score of 4, is at risk of stent underexpansion. B) Based on the high calcium score, atherectomy lesion preparation is performed, leading to a wire-biased debulking (white arrowheads) of the calcified lesion, reducing the minimal calcium thickness from 0.64 mm to 0.35 mm. C) High-pressure non-compliant balloon dilatation leads to fracture of the calcium at the point of thinning (white arrowhead), increasing the lumen area from 2.06 mm2 to 5.08 mm2. D) Following stent implantation there is further displacement of the fractured calcified segments leading to a final stent area of 9.38 mm2 at the point of maximal calcification and >100% stent expansion.
It is important to note the limitations of OCT in assessing calcified plaque, including the physical impedance for the imaging catheter to cross severely calcified lesions or to navigate tortuous vessels, and high light attenuation in very thick calcium not allowing visualisation of the outer border of the calcified plaque.
Zhang et al investigated the temporal changes in plaque morphology on OCT in non-culprit lesions in a group of patients who had undergone an initial PCI with baseline OCT assessment4.
The study consisted mainly of relatively younger patients (mean age 59 years) with a predominant initial presentation of acute coronary syndrome (~60%). It sought to assess the effects of medical therapy on non-culprit plaque progression over a relatively short follow-up of six months, during which time 50% of patients were on moderate-intensity statin and 36% on high-intensity statin, and 50% on an angiotensin-converting enzyme inhibitor or a beta-blocking agent.
The matched OCT pullbacks in the same vessel revealed interesting patterns of dynamic changes in plaque morphology in 42% of patients and 31% of non-culprit segments. While in 25% of patients these changes were considered favourable (from fibroatheromas to fibrous plaque or from thin-cap fibroatheromas [TCFAs] to fibroatheromas), in ~17% the reverse unfavourable changes occurred despite therapy. Similar bidirectional changes were observed at the segment-level analysis; thus, the overall distribution of the plaques was unaltered. Moreover, while mean fibrous cap thickness per patient increased, the minimum cap thickness was unchanged. Lastly, while there were no changes in lipid core arc, a tissue attenuation coefficient index (shown by the same group to reflect fibrotheroma and TCFA content) decreased over time.
The study by Zhang et al, despite its limitations as a single-centre retrospective study and its short longitudinal span of six months, provides interesting new observations on the vessel and plaque changes over time while on medical therapy. Perhaps the most striking finding was the dynamic nature of the changes in plaque morphology with both favourable and unfavourable changes occurring within the same vessel, adding more puzzles in the quest for the “vulnerable plaques” by OCT12. The study did not identify clinical or lesion characteristics that may independently predict such changes. Clearly, additional factors beyond reducing the systemic hypertension, lowering cholesterol levels and improving endothelial function afforded by current therapies are at play that may include local haemodynamics, inflammatory milieu, flow patterns and shear stress that may affect plaque progression or regression over time. Since no in vivo OCT imaging study has identified the substrates for plaque rupture or erosion, including establishing a relationship between the OCT-identified cap thickness and future events11, the significance of a lack of change in the minimum cap thickness per patient in this study remains unclear. Much larger, longer longitudinal studies using OCT, perhaps as part of a hybrid imaging strategy12, are needed to answer these essential questions.
The study by Usui et al sought to determine the correlation between OCT-determined minimum luminal area (MLA) and fractional flow reserve (FFR) in non-left main coronary arteries, a concept investigated in seven previous studies11, in a larger number of lesions/patients.
The study also compares the predictive value of OCT-MLA for FFR <0.75 or <0.8 to IVUS-determined MLA in the same lesions. As expected from the known discrepancy between IVUS and OCT measurements in vivo (with IVUS measurements being larger than those of OCT13), the OCT-MLA correlating with FFR <0.75 was lower than IVUS-MLA (1.39 mm2 vs. 2.57 mm2). The OCT-MLA cut-off value in this study was significantly smaller than in a previous study by Shiono et al (1.91 mm2) where OCT-MLA was correlated with FFR <0.7514, and perhaps expectedly lower than the values in other studies correlating OCT-MLA with FFR <0.8 (ranging from 1.59 to 2.05 mm2)11.
The authors show a better correlation between OCT-MLA and FFR <0.75 than with IVUS-MLA and FFR <0.75, whereas no such difference existed for lesions with FFR <0.8, an FFR threshold that is in use in clinical practice. Regardless of such comparisons, this study confirms the previously described modest positive and negative predictive values of both OCT-MLA and IVUS-MLA in determining the functional significance of lesions in non-left main coronary arteries11, reflecting additional factors beyond the degree of stenosis that determine functional significance, such as the amount of subtended viable myocardium. Thus, the decision whether to perform PCI on the basis of OCT-derived MLA alone can be erroneous in a significant proportion of lesions and is not recommended.
In conclusion, based on the three OCT-based studies published in EuroIntervention, OCT is a promising imaging modality in providing potentially impactful guidance on how to optimise the treatment of calcified coronary lesions. Identification of the vulnerable plaques, at least by OCT, remains elusive. The effects of current therapies on coronary plaques seem to vary in different segments of the vessel, indicating the need for a better mechanistic understanding of the underlying biological processes and more sophisticated imaging to elucidate novel ways to stabilise and regress plaques. Lastly, intracoronary imaging remains an important tool for stent optimisation but not in determining whether a lesion is flow-limiting and therefore indicated for PCI.
Conflict of interest statement
Z. Ali is a consultant for and holds grant support from St. Jude Medical. K. Galougahi has no conflicts of interest to declare.

