
Abstract
Aims: This was a retrospective study to develop and validate an optical coherence tomography (OCT)-based calcium scoring system to predict stent underexpansion.
Methods and results: A calcium score was developed using 128 patients with pre- and post-stent OCT (test cohort) and then validated in an external cohort of 133 patients. In the test cohort, a multivariable model showed that the independent predictors of stent expansion were maximum calcium angle per 180° (regression coefficient: –7.43; p<0.01), maximum calcium thickness per 0.5 mm (–3.40; p=0.02), and calcium length per 5 mm (–2.32; p=0.01). A calcium score was then defined as 2 points for maximum angle >180°, 1 point for maximum thickness >0.5 mm, and 1 point for length >5 mm. In the validation cohort, the lesions with calcium score of 0 to 3 had excellent stent expansion, whereas the lesions with a score of 4 had poor stent expansion (96% versus 78%, p<0.01). On multivariate analysis the calcium score was an independent predictor of stent underexpansion.
Conclusions: An OCT-based calcium scoring system can help to identify lesions that would benefit from plaque modification prior to stent implantation. Lesions with calcium deposit with maximum angle >180°, maximum thickness >0.5 mm, and length >5 mm may be at risk of stent underexpansion.
Abbreviations
AUC: area under the curve
IVUS: intravascular ultrasound
MSA: minimum stent area
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
Introduction
Stent underexpansion is the most robust predictor of stent restenosis and early thrombosis even with newer-generation drug-eluting stents1-3. Percutaneous coronary intervention (PCI) of calcified lesions is associated with a higher prevalence of target vessel revascularisation and myocardial infarction4,5, perhaps related to stent underexpansion due to severely calcified plaque6,7 and inadequate lesion preparation prior to stent implantation8. Current therapeutic options such as cutting/scoring balloon, rotational/orbital atherectomy, or excimer laser angioplasty may modify a severely calcified lesion before stent implantation; however, there are no clear guidelines to indicate the necessity for calcium modification9. Unlike intravascular ultrasound (IVUS), optical coherence tomography (OCT) can penetrate calcium to visualise calcium thickness. We hypothesised that a three-dimensional quantitative assessment of calcium – utilising the circumferential angle, thickness, and length – would predict stent underexpansion.
Methods
STUDY POPULATION
This was a retrospective, observational study to develop and validate an OCT-based calcium scoring system to predict stent underexpansion. This study consisted of two independent cohorts: the first (test) cohort was used to develop the calcium score, and the second cohort was used to validate the predictability of this calcium score on stent expansion. OCT-guided PCI was performed in both cohorts; however, the procedure was not defined by protocol, and interventional strategy was left to the operator’s discretion.
For the test cohort, 969 patients were screened between February 2016 and August 2016 to identify 200 who were enrolled in a randomised study to evaluate the utility of co-registration of OCT and angiography at St. Francis Hospital (Roslyn, NY, USA). Angiographic exclusion criteria for the original randomised study included: (i) left main disease; (ii) ostial lesion; (iii) tortuous artery in which OCT was unable to pass; (iv) bypass graft stenosis; (v) in-stent restenosis; or (vi) chronic total occlusion. In addition, lesions without either pre-stent or final OCT, without any calcium by OCT, or treated with rotational or orbital atherectomy or laser angioplasty were excluded from this study.
For the validation cohort, we screened patients with stable angina (to avoid the issue of thrombus obscuring underlying calcium) who underwent PCI between January 2013 and May 2015 at Tsuchiura Kyodo General Hospital (Ibaraki, Japan), using the same lesion exclusion criteria as the test cohort.
This study was approved by the ethics committees at St. Francis Hospital and Tsuchiura Kyodo General Hospital, and all patients provided written informed consent before participation.
OCT IMAGING ACQUISITION AND PCI
OCT was acquired by frequency-domain OCT (ILUMIEN™ C7-XR™ or OPTIS™) and Dragonfly™ Duo or OPTIS catheter (all Abbott Vascular, Santa Clara, CA, USA) using a non-occlusive technique. All patients underwent both pre-stent and final (post-stent) OCT. Pre-stent OCT was performed before intervention; however, if the OCT catheter did not pass the lesion, OCT was performed immediately after dilation using a small balloon. Moderate calcification was defined as radiopacities noted only during the cardiac cycle before contrast injection; severe calcification was defined as radiopacities observed without cardiac motion.
CORONARY ANGIOGRAPHY ANALYSIS
All angiograms were analysed using QAngio® (Medis, Leiden, the Netherlands) software and conventional definitions by two independent interventional cardiologists who were blinded to clinical and OCT information.
OCT ANALYSIS
All OCT data were analysed using ORW software version E.0.2 (Abbott Vascular) and expert consensus reports10,11 by two independent interventional cardiologists and reviewed by a third reader who were all blinded to the clinical and angiographic information.
EVALUATION OF CALCIFIED PLAQUE
Each calcium deposit in the target lesion was evaluated by pre-stent OCT using three parameters - maximum angle, maximum thickness, and length. When calcium was extremely thick and its border not clear due to attenuation, the maximum visible thickness was measured. Analysis was performed on a per-calcium deposit basis as well as on a per-target lesion basis. Individual calcium deposits within a single target lesion were separated by at least 1 mm of non-calcified plaque, and the total number of calcium deposits with an angle ≥30° was counted. Calcium thickness was analysed at the slice with the maximum angle. Calcium length was the total number of calcium-containing slices multiplied by the frame interval. Finally, if there was more than one calcium deposit, the one with the largest maximum calcium angle was chosen to represent target lesion calcium, and its maximum angle, maximum thickness, and length were representative of each lesion.
ENDPOINT
The endpoint was final post-PCI stent expansion (by OCT, based on the smallest stent area divided by the average of proximal and distal reference lumen area × 100) within the target lesion calcium.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and were compared using χ2 statistics or Fisher’s exact test, as appropriate. Continuous variables are presented as median and first and third quartiles and were compared using Wilcoxon’s test or the Kruskal-Wallis rank-sum test, as appropriate. Receiver operating characteristics with area under the curve (AUC) were used to determine the best cut-off value for maximum calcium angle, maximum thickness, and length predicting stent underexpansion. Based on their historical and mechanistic relationship to stent expansion, other potential predictive factors were included in the multivariate linear regression model for predicting stent underexpansion. Cohen’s kappa coefficient was used to assess inter- and intra-observer variability of the OCT-based calcium score using 30 randomly selected cases by two independent observers and by a single observer’s re-analysis one month later. Two-sided p-values <0.05 were used to indicate statistical significance. Statistical analyses were performed using JMP version 8.0 (SAS Institute Inc., Cary, NC, USA).
Results
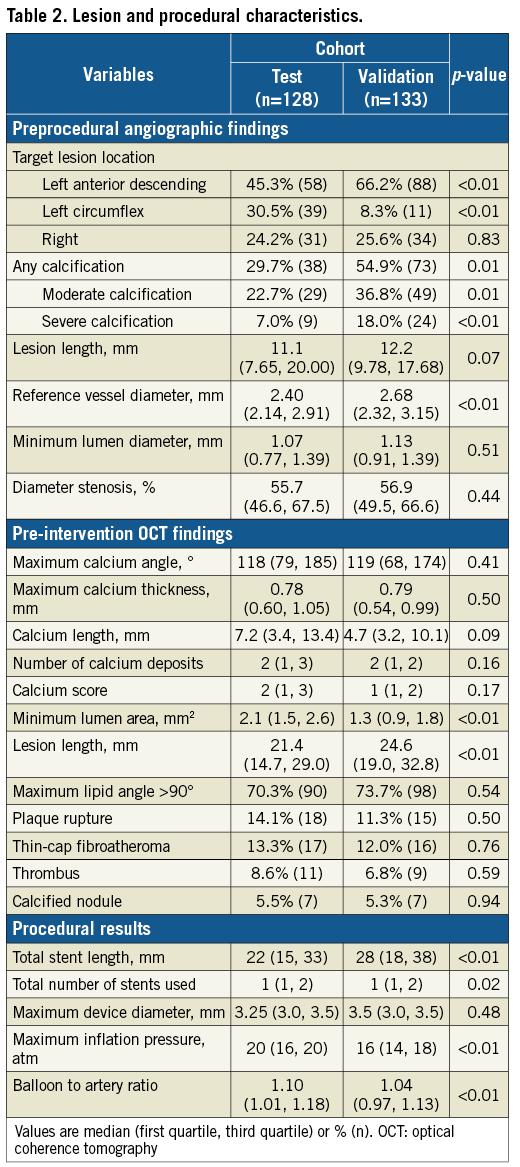
PATIENT, LESION, AND PROCEDURAL CHARACTERISTICS IN TEST AND VALIDATION COHORTS
For the test cohort, of the 200 patients enrolled in the OCT co-registration study, four lacked either pre-stent or final OCT, 17 were treated with orbital atherectomy, and 51 had no calcium in the final OCT and were therefore excluded. No patients were treated with rotational atherectomy, laser, or cutting or scoring balloon. Finally, 128 patients (128 calcified lesions) were included (Figure 1).
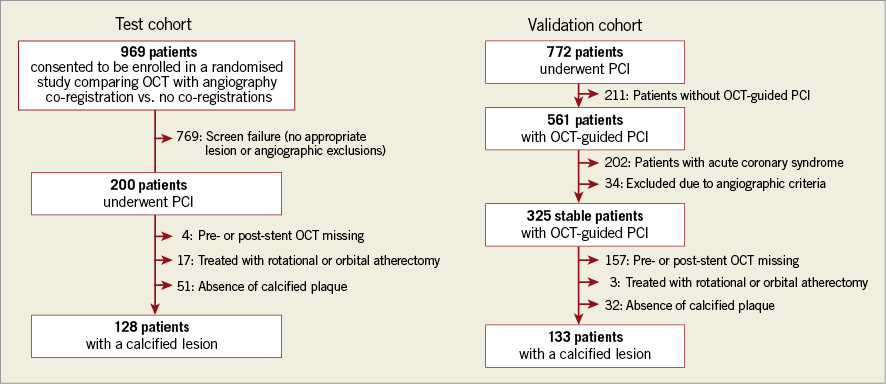
Figure 1. Study flow chart of the test and validation cohorts.
For the validation cohort, 561 of 772 patients who underwent OCT-guided PCI were assessed for eligibility. Of these, 202 patients with acute coronary syndromes, 34 who met angiographic exclusion criteria, 157 without either pre-stent or final OCT, three treated with rotational atherectomy, and 32 without any calcium in the final OCT were excluded. No patients were treated with orbital atherectomy, laser, or cutting or scoring balloon. Finally, 133 patients (133 calcified lesions) were selected. The prevalence of patients without calcified plaque was greater in the test cohort (51/200) than in the validation cohort (32/325), probably because of a difference in baseline patient characteristics (i.e., higher prevalence of diabetes mellitus, current smoking, and stable coronary artery disease in the validation cohort).
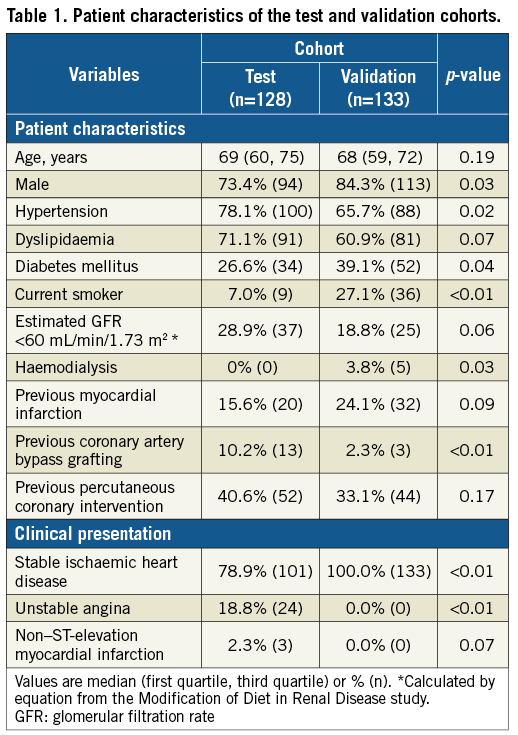
Comparisons of baseline patient and lesion/procedural characteristics between the two cohorts are shown in Table 1 and Table 2. The prevalence of an estimated glomerular filtration rate <60 mL/min/1.73 m2 was not different between the two cohorts. There were five patients with haemodialysis in the validation cohort, but none in the test cohort. Patients in the validation cohort had more severely calcified lesions assessed by angiography than in the test cohort. Minimum lumen area was significantly smaller in the validation cohort, although percent diameter stenosis was similar in the two cohorts, possibly because lesion calcification caused angiographic haziness to underestimate lesion severity when working with a limited number of angiographic views. Patients in the test cohort had shorter total stent length, a larger number of stents, higher maximum inflation pressures, and larger balloon-to-artery ratios. Although the maximum device diameter was greater than the reference diameter assessed by angiography in both cohorts, the balloon-to-artery ratio based on OCT was appropriate (1.10 for the test cohort; 1.04 for the validation cohort). Lesion length assessed by angiography was shorter than assessed by OCT, probably due to underestimation of lesion length by the angiogram, because lesion length assessed by OCT (21.4 mm in the test cohort and 24.5 mm in the validation cohort) was compatible with total stent length (22 mm in the test cohort and 28 mm in the validation cohort).
SCORE DEVELOPMENT
Based on the test cohort, we developed a calcium scoring system using a multivariate linear regression model to predict stent expansion. Maximum calcium angle, maximum calcium thickness, calcium length, number of calcium deposits, total stent length, maximum balloon pressure, and balloon-to-artery ratio were included as potential factors in the multivariable model. Maximum calcium angle, maximum calcium thickness, and calcium length were found to be independent predictors for stent expansion (Table 3). Using receiver operating curves, the best cut-off values of each parameter to predict stent expansion <70% were: (i) 188° for maximum calcium angle; (ii) 0.75 mm for maximum calcium thickness, and (iii) 7.1 mm for calcium length. Based on these results, we assigned 1 or 2 points to each of three conditions: 2 points for maximum calcium angle >180°, 1 point for maximum calcium thickness >0.5 mm, and 1 point for calcium length >5 mm (Figure 2, Figure 3). There was a good concordance of inter- and intra-observer agreement for assessment of the maximum calcium angle (κ=0.86 and 0.93, respectively), maximum calcium thickness (κ=0.63 and 0.71, respectively), calcium length (κ=0.73 and 0.86, respectively), and calcium score (κ=0.83 and 0.91, respectively).
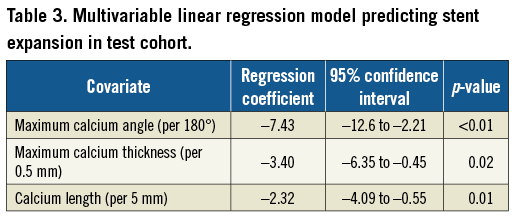
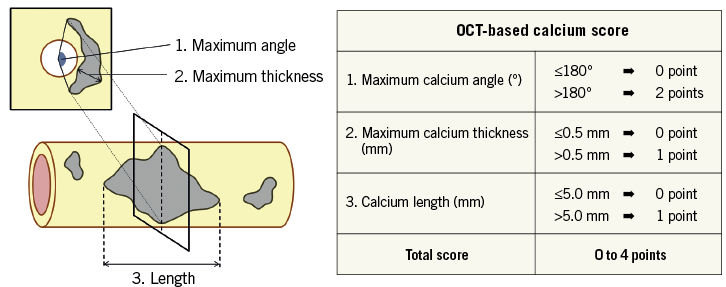
Figure 2. Optical coherence tomography-based calcium scoring system. OCT-based calcium score was composed of three parameters that were derived from pre-stent OCT: (i) maximum calcium angle; (ii) maximum thickness; and (iii) calcium length. One or two points were assigned for each parameter, and the total score (0 to 4) calculated. OCT: optical coherence tomography
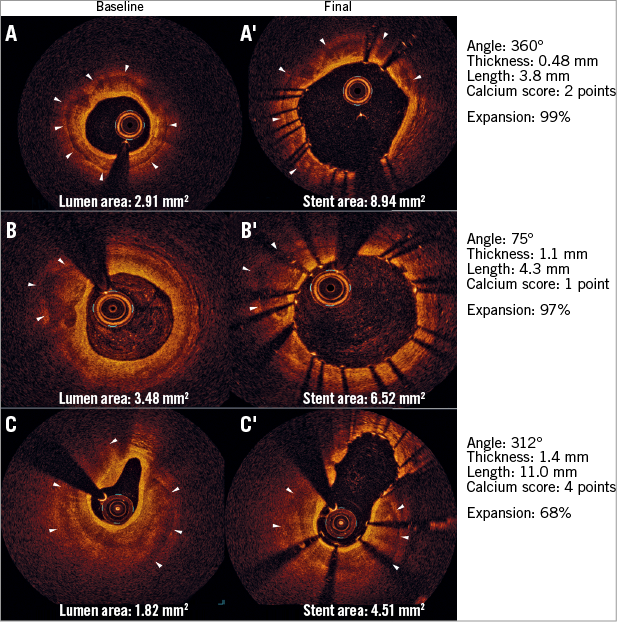
Figure 3. Association between calcium score and stent expansion. Case A. Calcium score of 2 (large-angled and thin calcium) and excellent stent expansion. Case B. Calcium score of 1 (small-angled and thick calcium) and excellent stent expansion. Case C. Calcium score of 4 with stent underexpansion.
VALIDATION OF CALCIUM SCORING SYSTEM
Table 4 shows pre- and post-intervention findings and procedural results in the validation cohort. Validation cohort lesions with a calcium score of 0, 1, 2, or 3 had adequate stent expansion. On the other hand, 24 lesions with a calcium score of 4 showed poor stent expansion (median 78%). Although the endpoint of this study was stent expansion “within target lesion calcium”, there was also a stepwise decrease in stent expansion at the minimum stent area (MSA) site that was associated with an increasing calcium score; stent expansion at the MSA site among lesions with a calcium score of 4 was poor (median 69%). Figure 4 shows the prevalence of stent expansion <70%, suggesting that each of the three parameters of the calcium scoring system contributed to the prediction of stent underexpansion.
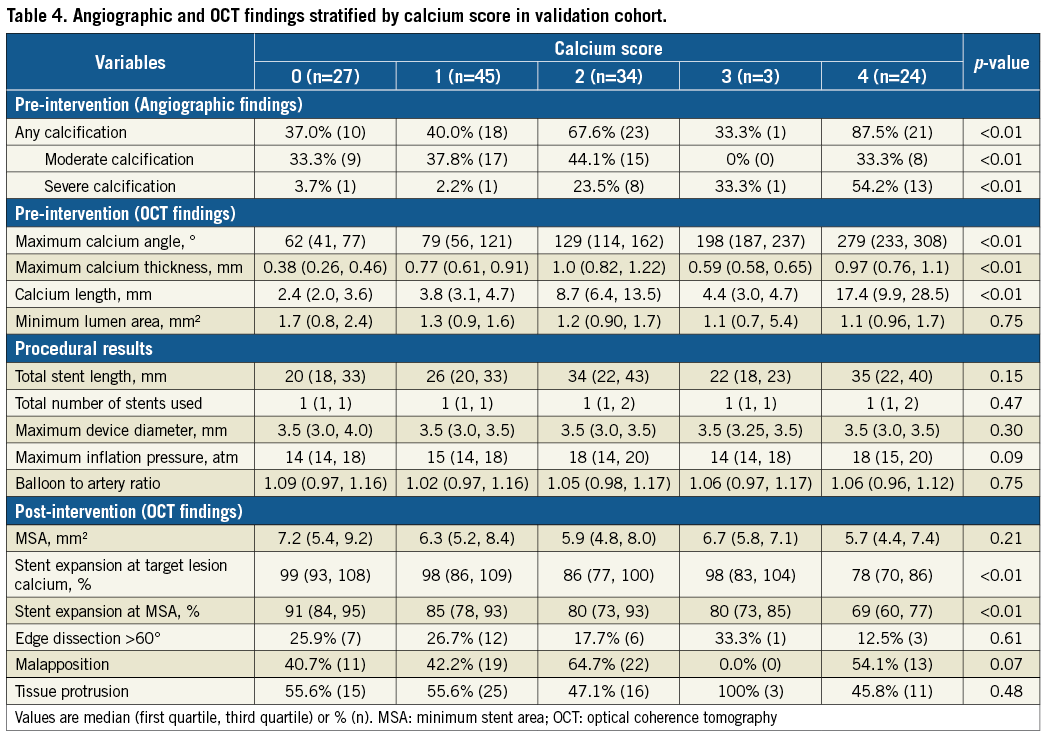
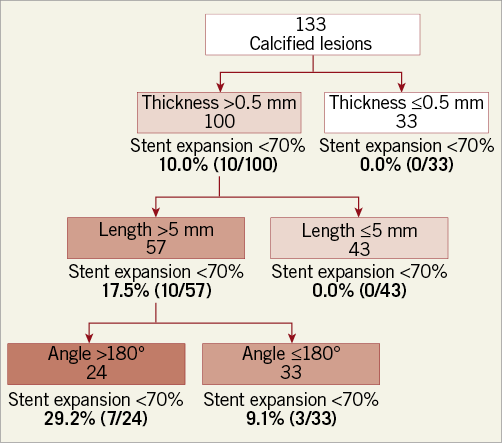
Figure 4. Prevalence of stent expansion <70% on the basis of calcium angle, thickness, and length in the validation cohort. Among 100 (of 133) lesions with calcium thickness >0.5 mm, 10.0% (10/100) had stent expansion <70%. Among 57 (of 100) lesions with calcium thickness >0.5 mm and calcium length >5 mm, 17.5% (10/57) had stent expansion <70%. Among 24 (of 57) lesions with maximum thickness >0.5 mm, length >5 mm, and maximum angle >180°, 29.2% (7/24) had stent expansion <70%.
In a multivariate linear regression model that included the calcium score, number of calcium deposits, total stent length, maximum balloon pressure, and balloon-to-artery ratio as covariates, calcium score was an independent predictor of stent underexpansion (regression coefficient –5.68, 95% confidence interval [CI]: –7.54 to –3.81, p<0.01).
Within 87.2% of the lesions, the smallest stent area within target lesion calcium was located at the site of the maximum calcium angle. Approximately one third of lesions had an MSA that was smaller than the smallest stent area within the target lesion calcium, mainly because the true MSA was located at the distal end of the lesion due to vessel tapering.
Notably, the prevalence of moderate to severe calcification assessed by angiography was significantly increased with a greater calcium score. One fourth of the lesions with a calcium score of 4 (6/24) and severe calcification assessed by angiography (6/24) had stent expansion <70%. The AUCs to predict stent expansion <70% were 0.86 (calcium score) and 0.84 (angiogram), respectively (p=0.79). The multivariable linear regression model including angiographic calcium assessment (none/mild, moderate, and severe) demonstrated that angiographic assessment was also an independent predictor of poor stent expansion (regression coefficient –7.89, 95% CI: –13.19 to –2.60, p<0.01).
Discussion
The principal findings of this study are the following. (i) Using the results of a multivariate analysis predicting stent expansion, a calcium scoring system was developed that included calcium angle, thickness, and length, but not the number of calcifications. The amount of calcium that impacted on stent expansion was defined by the single largest deposit of target lesion calcium. (ii) The utility of the calcium scoring system to predict stent underexpansion was then confirmed in a separate validation cohort in which lesions with a calcium score of 4 had a particularly high risk of stent underexpansion. (iii) There was good concordance of inter- and intra-observer agreement for the calcium score, indicating that it was highly reproducible.
STRATEGY OF PCI WHEN TREATING CALCIFIED LESIONS
Stent underexpansion is observed in about half of in-stent restenosis; treating stent underexpansion in a heavily calcified lesion is more difficult than preventing underexpansion12. Optimal lesion preparation prior to stent implantation increases the likelihood of a favourable clinical outcome13. Clinical guidelines recommend the use of rotational atherectomy for preparation of heavily calcified lesions that cannot be crossed by a balloon or adequately predilated before stent implantation8; however, a randomised controlled trial of patients with moderate to severe angiographic calcium failed to demonstrate clinical benefit of rotational atherectomy before paclitaxel-eluting stent implantation compared with balloon predilation alone14. Other studies have suggested the potential benefits of orbital atherectomy or laser angioplasty for calcified lesions12,15. Therefore, it is important to identify lesions that need plaque modification before stent implantation.
THE POTENTIAL UTILITY OF OCT TO EVALUATE CALCIFIED PLAQUE
The evaluation of calcium by IVUS is limited because high-intensity reflection with acoustic shadowing precludes assessment of thickness. In contrast, because it can penetrate calcium, measure calcium thickness, and assess the three-dimensional extent of calcium, OCT has the potential to assess calcified plaque and quantify the amount of calcium better than IVUS16. Although OCT assessment of deep calcium as well as precise measurements of very thick calcium deposits might be limited, OCT provides precise evaluation for superficial calcification that could impact on stent expansion; however, previous OCT studies have not indicated the amount of calcium that might prompt the interventional cardiologist to consider plaque modification pre-stenting. The current OCT-based calcium scoring system has the potential to be an easy, practical and reproducible tool to identify lesions that would benefit from pre-stent plaque modification versus balloon predilation alone. Our study indicated that the risk of stent underexpansion was increased in lesions with a calcium score of 4; thus, aggressive lesion modification should be considered when treating calcified lesions with a maximum angle >180°, maximum thickness >0.5 mm, and length >5 mm.
Recently, Wang et al reported that detection of calcium by coronary angiography correlated with that by intravascular imaging17. Our study showed similar predictability for stent underexpansion comparing severe angiographic calcium and an OCT calcium score of 4. However, this was based on six patients with stent underexpansion among 24 patients with severe calcium in the overall validation cohort of 133 patients. OCT provides additional information including location (deep versus superficial) of calcium, allows quantification with better reproducibility, is not affected by body mass, and can identify calcium fractures that are associated with better expansion even in severely calcified lesions.
Study limitations
First, this was a retrospective, observational study with a modest number of patients. Second, our cohort did not include severely calcified lesions that could not be crossed with the OCT catheter pre-stenting. Third, our cohort included relatively simple calcified lesions for which we would not normally consider lesion modification; nevertheless, even within these lesions, this calcium scoring system had important predictive value. The angiographic and OCT characteristics of the patients enrolled in this study oriented towards a low-intermediate lesion/patient complexity (e.g., intermediate % diameter stenosis, less than 180° of maximum calcium angle and relatively shorter calcium length). Fourth, our cohort included few patients with haemodialysis; thus, it is uncertain that our results can be applied to patients with renal failure. Finally, the endpoint was stent expansion at the smallest stent area within the target lesion calcium instead of at the MSA site; however, the findings were similar when analysing the MSA site.
Conclusions
This OCT-based calcium scoring system may be useful to identify calcified lesions that would benefit from plaque modification prior to stent implantation. Lesions with calcium score of 4 (calcium deposit with maximum angle >180°, maximum thickness >0.5 mm, and length >5 mm) are at risk of stent underexpansion. The advantage of OCT compared to angiography alone should now be tested in a larger and more heterogeneous cohort of patients.
| Impact on daily practice Lesions with calcium deposit with maximum angle >180°, maximum thickness >0.5 mm, and length >5 mm assessed by OCT are at risk of stent underexpansion. |
Acknowledgements
The authors thank Yoshihisa Kanaji, MD, Tadashi Murai, MD, Allen Jeremias, MD, Ziad Ali, MD, DPhil, Sosa Fernando, MS, Dominic P. Francese, MPH, and Jeffery W. Moses, MD, for their help in this study.
Conflict of interest statement
A. Fujino has received grant support from Asahi Intecc. G. Mintz has received grant support and is a consultant for Volcano, Boston Scientific, ACIST, Infraredx, and Abbott Vascular. A. Maehara has received institutional grant support from Abbott Vascular, and Boston Scientific, and is a consultant for Boston Scientific, and OCT Medical Imaging Inc. The other authors have no conflicts of interest to declare.

