
Abstract
Aims: The aim of this study was to use optical coherence tomography (OCT) to predict newly implanted stent expansion for treatment of in-stent restenosis (ISR).
Methods and results: With OCT guidance, 143 ISR lesions were treated with a new stent. Stent underexpansion was defined as minimum stent area (MSA) <4.5 mm2 and MSA/average of reference lumen area <70%. New stent underexpansion was found in 33 lesions (23%). These had a smaller old stent MSA (4.13 [3.32-4.62] versus 5.18 [4.01-6.38] mm2, p=0.001), and had a higher prevalence of multiple old stent layers (51.5% versus 10.9%, p<0.001) and neointimal or peri-stent calcium (69.7% versus 37.3%, p=0.001) compared to those without new stent underexpansion. Old stent underexpansion, multiple layers of old stent, maximum calcium angle >180°, and maximum calcium thickness >0.5 mm were independently associated with new stent underexpansion. Patients with new stent underexpansion had a higher prevalence of major adverse cardiac events (35.5% vs 14.3%, p=0.009), mainly driven by a higher rate of myocardial infarction and target vessel revascularisation at two years.
Conclusions: When re-stenting an ISR lesion, old stent underexpansion, the amount of neointimal or peri-stent calcium, and multiple old stent strut layers are important determinants of new stent underexpansion which is then associated with adverse long-term outcomes.
Introduction
Although advances in drug-eluting stents (DES) have substantially reduced the risk of coronary in-stent restenosis (ISR) and the need for target lesion revascularisation, ISR persists1,2. There are several treatment options for ISR (conventional balloon angioplasty, cutting or scoring balloons, drug-coated balloons, or bypass surgery); however, repeat DES implantation has superior clinical and angiographic outcomes to other treatment strategies3. Nevertheless, there are few data which evaluate the morphological predictors of new stent underexpansion when treating an ISR lesion. We hypothesised that the morphological characteristics underlying the ISR lesion, i.e., neoatherosclerotic or peri-stent calcium, would impact on the expansion of a newly implanted stent and that these morphologic characteristics could be evaluated with optical coherence tomography (OCT).
Methods
PATIENT POPULATION
This was a retrospective, observational study to assess morphological factors that contributed to new stent underexpansion when treating ISR lesions using OCT guidance at two hospitals (New York-Presbyterian Hospital, New York, NY, USA; St. Francis Hospital, Roslyn, NY, USA). The indication of treatment (symptoms and/or evidence of ischaemia) and treatment strategy were at each operator’s discretion. Patients with pre-OCT and final (post new stent implantation) OCT evaluation were enrolled. The research protocol was approved by the ethics committee of each hospital. Clinical follow-up was performed by hospital chart review, outpatient clinic visit, or telephone contact. Major adverse cardiac events (MACE) were a composite of all-cause death, myocardial infarction (MI), or clinically driven target vessel revascularisation (TVR).
Coronary angiograms were analysed by an interventional cardiologist (D. Yin) at the Cardiovascular Research Foundation (New York, NY, USA) using QAngio XA version 7.2 (Medis medical imaging systems, Leiden, the Netherlands) using conventional methods4. Angiographic restenosis was classified as: (i) focal ISR (<10 mm in length); (ii) diffuse ISR (>10 mm length, but within the stent); (iii) proliferative (>10 mm in length, extending beyond the stent edges); and (iv) total occlusion5.
OCT IMAGING AND ANALYSIS
Pre-intervention, an OCT catheter (C7 Dragonfly™ or Dragonfly™ Duo; Abbott Vascular, Santa Clara, CA, USA) was introduced distal to the lesion, and contrast media was injected via the guiding catheter at 3-4 mL/s during pullback. Pre-OCT predilation with a 1.5-2.0 mm balloon was performed in severe ISR. OCT images were acquired using frequency-domain OCT (C7-XR™, ILUMIEN™, or ILUMIEN™ OPTIS™; Abbott Vascular) with a frame interval of 0.1-0.2 mm6. After successful re-stenting, OCT imaging was repeated. Off-line analysis was performed by agreement of two independent cardiologists (D. Yin and A. Maehara) using proprietary software (Abbott Vascular).
All OCT slices were evaluated; stent and intra-stent lumen cross-sectional areas (CSA) were measured at the minimum lumen CSA, minimum stent CSA (MSA), and maximum neointimal hyperplasia (NIH) CSA. Stent CSA was measured by joining strut blooming middle points. If the stent was covered by high signal attenuation tissue, stent CSA was interpolated using proximal and distal slices. Percentage of NIH was calculated as (1–lumen/stent CSA)×100. Proximal and distal reference lumen CSAs were at the slices with the largest lumen CSA within 5 mm proximal and distal to the stent edges, but before significant side branches (>1.5 mm in diameter). Stent expansion was MSA divided by the average of the proximal and distal reference lumen CSA. Stent underexpansion was MSA <4.5 mm2 and stent expansion <70% based on the CLI-OPCI II study7.
Calcium was a region with a well-delineated border and subcategorised as either within the NIH or native plaque behind the stent (Figure 1A),8. Maximum calcium angle (sum of angle in each slice) and maximum calcium thickness were measured, and lengths were calculated by the total slice number multiplied by the frame interval. Calcium fracture was defined as complete discontinuity of calcified plaque (Figure 1B). Double old stent layers were two layers of old stent struts within the same OCT frame (Figure 1C). There was no lesion with more than two old stent layers. Tissue protrusion, stent malapposition, and edge dissection were also assessed post re-stenting9.
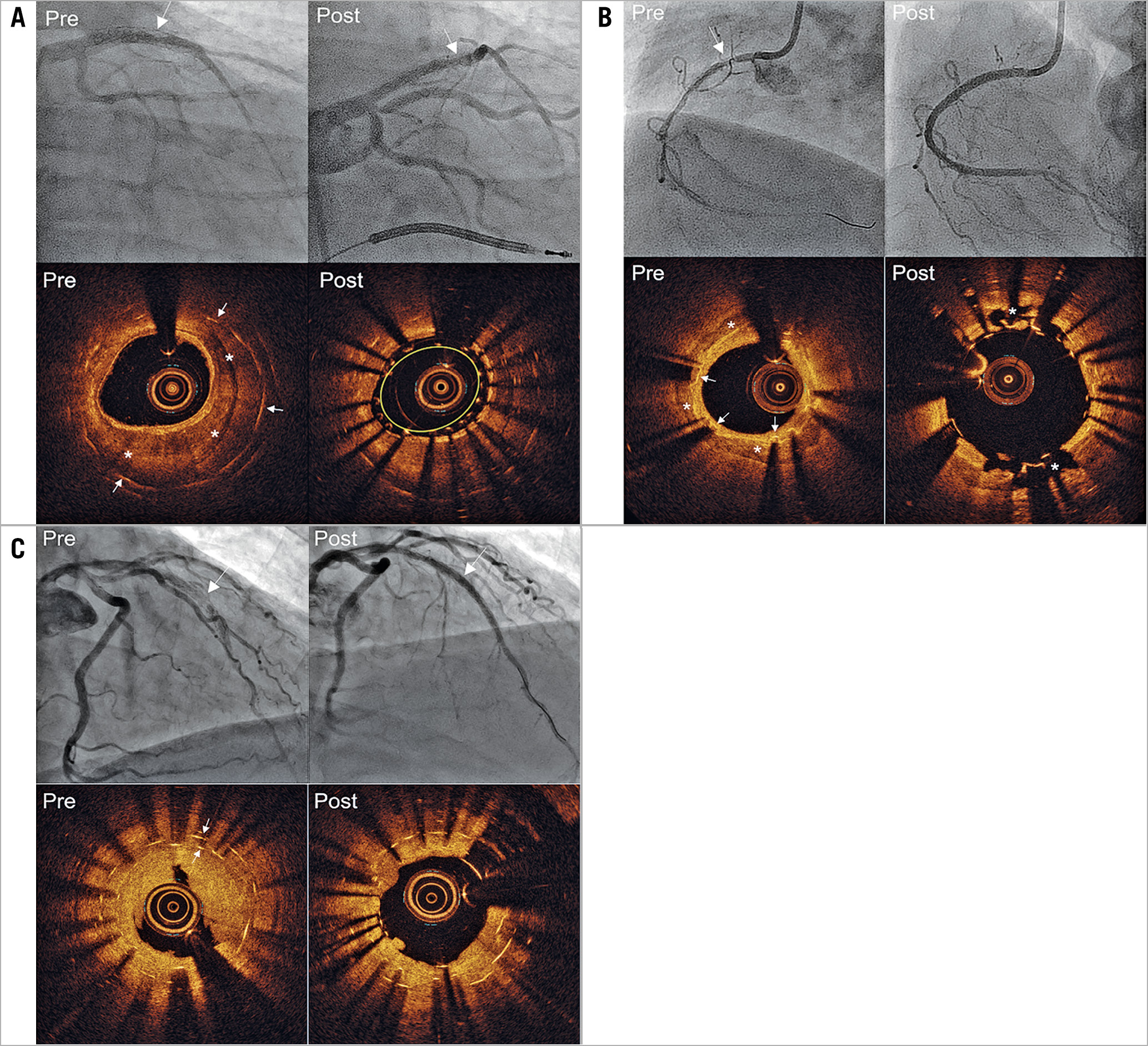
Figure 1. Angiography and optical coherence tomography examples of re-stenting in-stent restenosis lesions. A) Neointimal calcium and re-stent underexpansion. The angiogram shows in-stent restenosis (arrow) in the middle of the left anterior descending coronary artery (LAD), and pre-procedure optical coherence tomography (OCT) shows neointimal calcium (asterisks) within the old stent (arrows) with minimum lumen area (MLA)=1.69 mm2 and minimum stent area (MSA)=4.38 mm2. Post-re-stenting angiogram and OCT show new stent underexpansion (MSA=1.86 mm2, yellow circle). B) Re-stenting with good expansion due to calcium fracture. The angiogram shows in-stent restenosis (arrow) in the proximal right coronary artery, and the pre-procedure OCT shows calcium (asterisks) behind the old stent (arrows) with MLA=2.33 mm2 and MSA=3.07 mm2. Post-re-stenting angiogram and OCT show calcium fracture (asterisks) with MSA=5.04 mm2. C) New stent underexpansion due to two old stent layers. The angiogram shows in-stent restenosis (arrow) in the middle of the LAD, and pre-procedure OCT shows two old stent layers (arrows) with MLA=1.15 mm2 and MSA=3.25 mm2 (inner layer). Post-re-stenting angiogram and OCT show new stent underexpansion with MSA=2.43 mm2.
STATISTICAL ANALYSIS
Normally distributed continuous variables were reported as mean and standard deviation and compared using the Student’s t-test. Non-normally distributed continuous variables were reported as median with interquartile range and compared using the Mann-Whitney U test. Categorical variables were summarised as counts and percentages and compared using χ2 statistics or Fisher’s exact test. Receiver operating characteristic (ROC) curves were used to determine cut-off values (Youden index) for maximum calcium angle and thickness associated with new stent underexpansion. A multivariable logistic regression model was performed to identify factors associated with new stent underexpansion. Included variables were chosen based on their historical or mechanistic relationship to stent underexpansion10,11,12. Time-to-first-event rates are shown as Kaplan-Meier estimates and compared with the log-lank test; p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
CLINICAL CHARACTERISTICS
From February 2011 to March 2017, 655 lesions (633 patients) underwent OCT evaluation for ISR: 344 lesions did not have an intervention, 62 were treated by balloon angioplasty only, 13 were treated with atherectomy, 58 did not have final OCT, 35 lesions had poor OCT quality, leaving 143 ISR lesions (143 patients were enrolled). Duration from implantation was 5.8±4.8 years and was >5 years in 50.7%. Ninety-four had acute coronary symptoms, 30 had stable angina, and 16 had a positive stress test without symptoms. Based on final post-re-stenting OCT measurements, 33 lesions had an MSA <4.5 mm2 and stent expansion <70% and were considered to have new stent underexpansion; the rest comprised the comparison group (n=110) (Figure 2). There were no differences in clinical characteristics between the groups (Table 1).
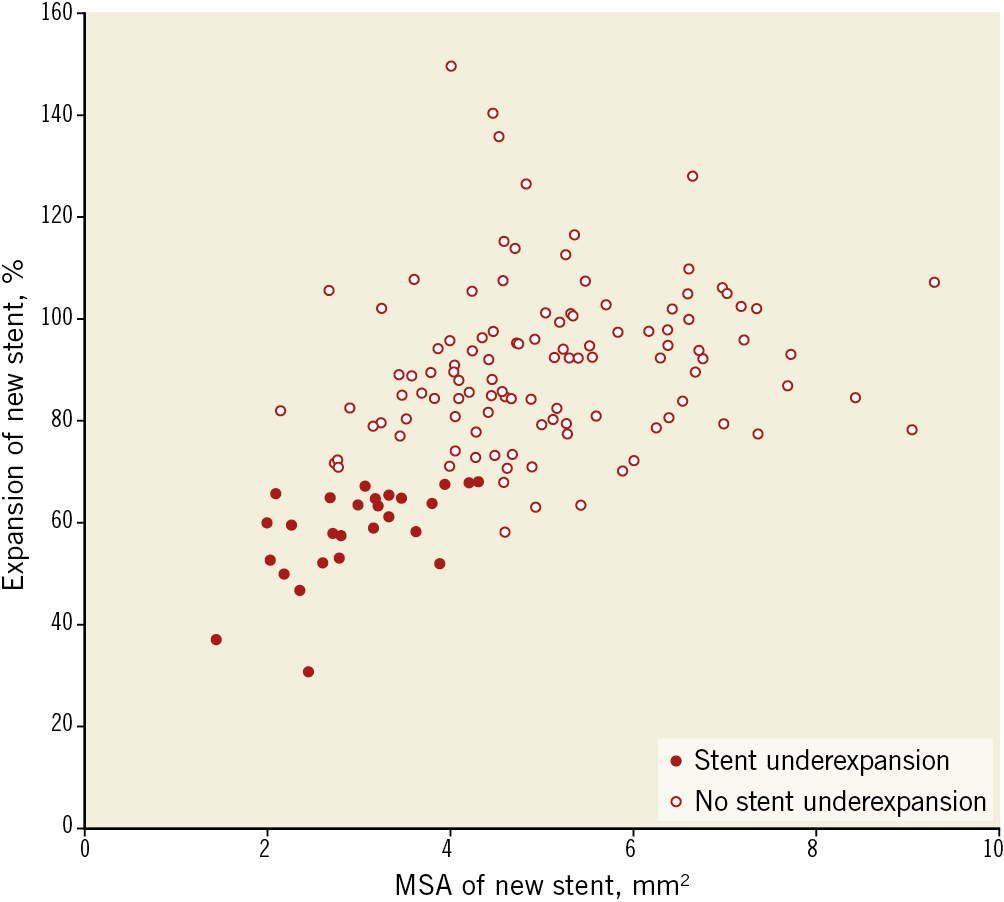
Figure 2. Minimum stent area and expansion of a new stent. A new stent with both a minimum stent area (MSA) <4.5 mm2 and expansion <70% was defined as stent underexpansion.
PROCEDURAL CHARACTERISTICS AND ANGIOGRAPHIC FINDINGS
Restenotic stents included bare metal stents (12.6%), first-generation DES (30.8%), and second-generation DES (56.6%). Predilation with a scoring balloon or a non-compliant balloon, maximum post-dilation pressure, and balloon/artery ratio were similar between the two groups. All but two ISR lesions were then treated using second-generation DES; new stent diameter (2.75 [2.5-3.0] versus 3.0 [2.75-3.5] mm, p=0.001) and maximum post-dilation balloon diameter (3.0 [2.75-3.38] versus 3.25 [3.0-3.5] mm, p=0.009) were smaller in patients with versus without new stent underexpansion (Table 2). As shown in Table 3, angiographic pre-PCI and final post-PCI in-stent dimensions and acute gain were not significantly different between the groups.
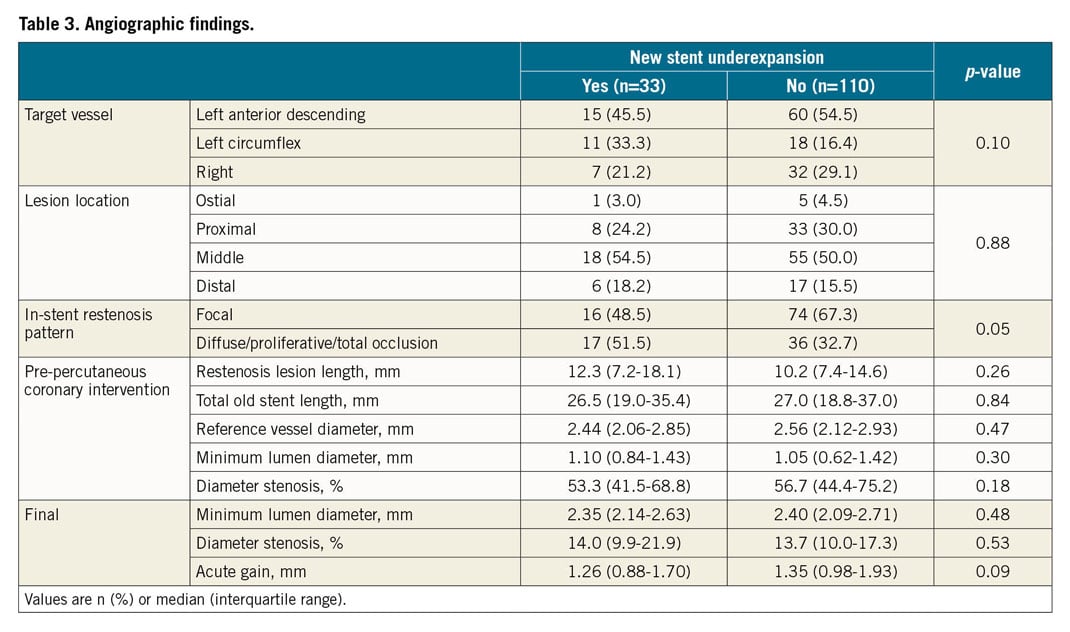
OCT FINDINGS
Based on pre-intervention OCT, old stent underexpansion (old stent MSA <4.5 mm2 and old stent expansion <70%) were identified in 12.6% (18/143), in-stent neoatherosclerosis was identified in 48.3% (69/143), and the rest of the ISR lesions were mainly due to NIH (i.e., no old stent underexpansion, no neoatherosclerosis, 39.2%, 56/143). Lesions with new stent underexpansion had a smaller old stent MSA (4.13 [3.32-4.62] versus 5.18 [4.01-6.38] mm2, p=0.001), and the mechanism of old stent failure was more often underexpansion (39.4% versus 4.5%, p<0.001) (Table 4). The prevalence of a double layer of old stents was higher in lesions with new stent underexpansion (51.5% versus 10.9%, p<0.001). There was no difference in new stent malapposition, stent tissue protrusion, or stent edge dissection between the two groups.
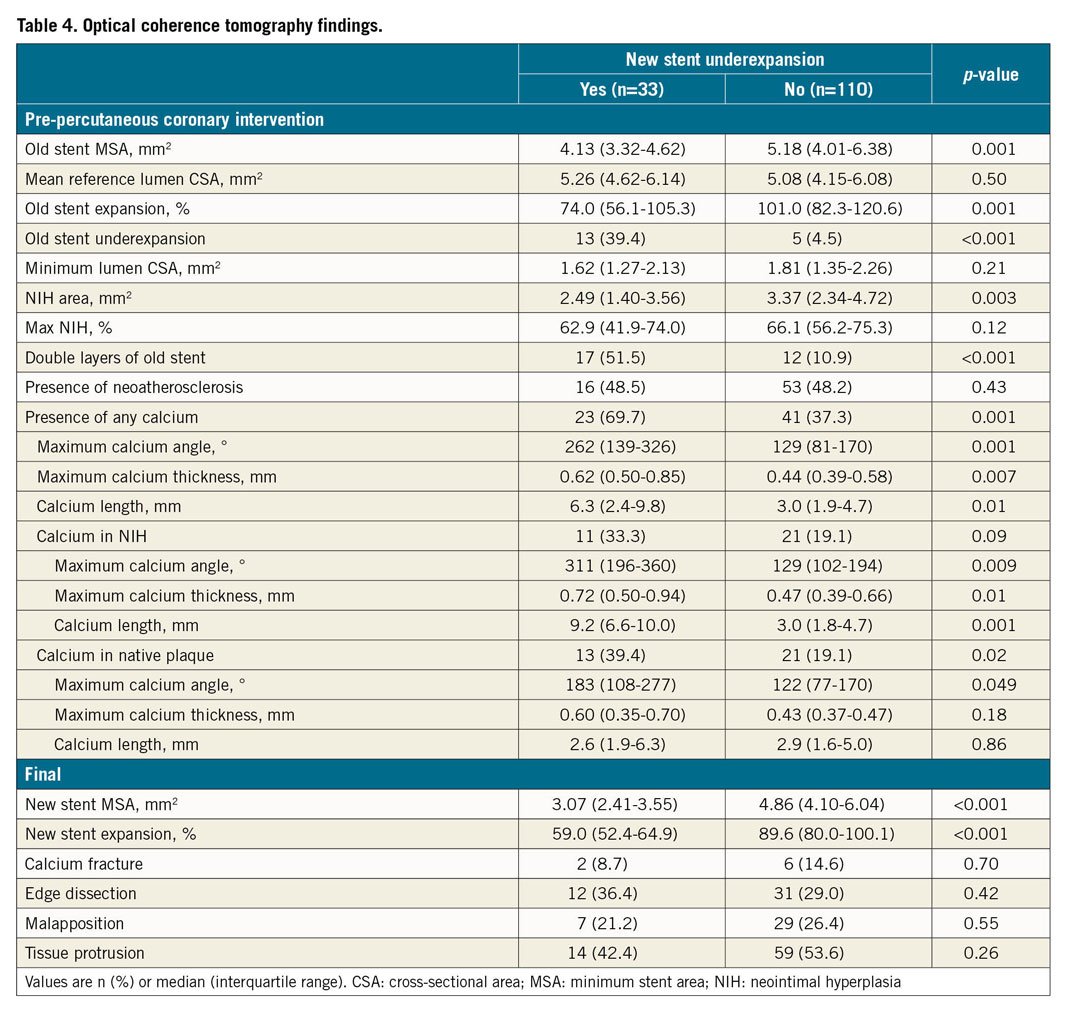
Calcium, including neointimal calcium or calcium in the plaque behind the old stent, was more common in new stent underexpansion (69.7% versus 37.3%, p=0.001) along with a larger angle and greater thickness, especially neointimal calcium (Table 4). Using ROC analysis, the cut-off value to predict new stent underexpansion was maximum calcium angle (either neointimal calcium or calcium behind stent) of 177° (area under the curve [AUC] 0.75, 95% confidence interval [CI]: 0.62-0.88, p=0.001, sensitivity=68%, specificity=78%) and maximum calcium thickness (either neointimal calcium or calcium behind stent) of 0.49 mm (AUC 0.71, 95% CI: 0.56-0.86, p=0.007, sensitivity=81%, specificity=68%), especially when the two co-existed (Figure 3). Calcium fracture post PCI was seen in only 8.7% (2/23 with calcium) in the new stent underexpansion group versus 14.6% (6/41 with calcium) that did not have new stent underexpansion. Among eight cases with calcium fracture post re-stenting, five fractures were in neointimal calcium, and three fractures were in calcium in plaque behind the old stent.
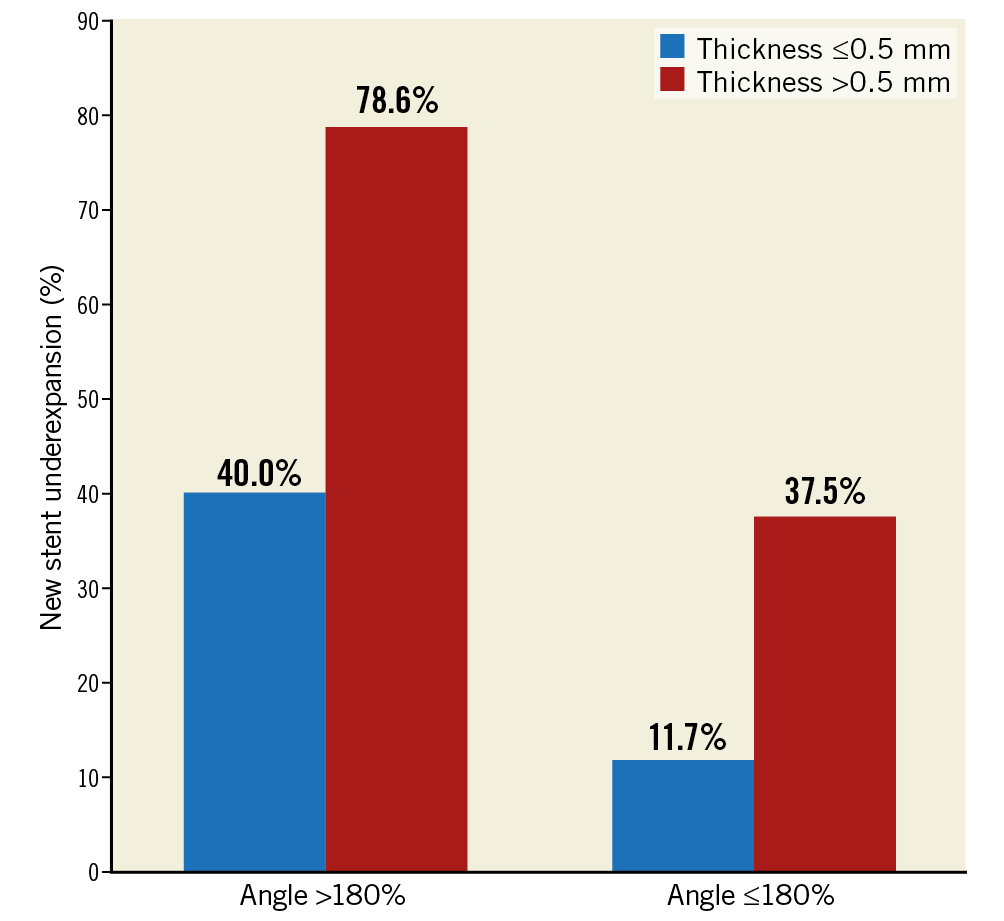
Figure 3. Prevalence of new stent underexpansion in in-stent restenosis lesions stratified by maximum calcium thickness and maximum calcium angle. Calcium angle >180° and calcium thickness >0.5 mm are additive in causing stent underexpansion.
PREDICTORS OF NEW STENT UNDEREXPANSION
In the multivariable analysis, old stent underexpansion (odds ratio [OR] 6.19, 95% CI: 1.82-7.61, p=0.006), double layers of old stent (OR 8.62, 95% CI: 2.15-13.3, p<0.001), calcium >180° (OR 5.80, 95% CI: 1.76-7.84, p=0.005), and maximum calcium thickness >0.5 mm (OR 4.83, 95% CI: 1.58-6.81, p=0.009) were independently associated with new stent underexpansion when re-stenting an ISR lesion. When different definitions of stent underexpansion were used, predictive factors remained consistent (Supplementary Table 1).
LONG-TERM OUTCOMES
Patients with new stent underexpansion had a higher prevalence of MACE, mainly driven by a higher rate of MI and TVR compared to no new stent underexpansion (Table 5, Figure 4).
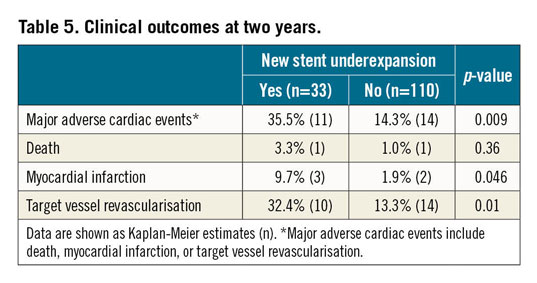
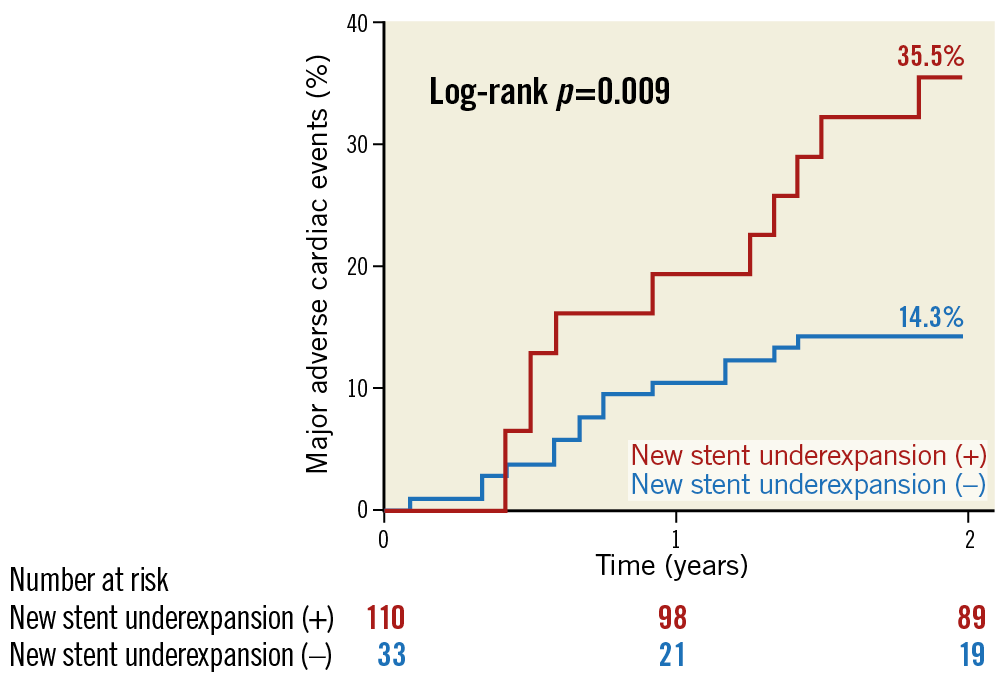
Figure 4. MACE rates between patients with versus without new stent underexpansion. Patients with new stent underexpansion had more than twice the event rate versus those without new stent underexpansion (35.5% versus 14.3%, p=0.009) at two years. MACE: major adverse cardiac events
Discussion
The present OCT study demonstrated that ISR lesions that were associated with old stent underexpansion, significant neointimal or peri-stent calcium (based on thickness and angle), and multiple layers of old stent struts were associated with new stent underexpansion when re-stenting the ISR lesion and that new stent underexpansion was associated with a higher event rate at follow-up.
Stent underexpansion, a main mechanism of ISR8, contributed to new stent underexpansion in the present study even when higher pressures or larger balloons were used13. An intravascular ultrasound study showed that acute diameter gain decreased with increasing arc of calcification14. As expected, it was difficult to expand a new stent within an underexpanded old stent due to calcified plaque.
Severe calcification in de novo coronary arteries limits stent expansion9,10,11,12,13. Kobayashi et al10 assessed the relationship between stent expansion and coronary calcification using OCT and demonstrated that a larger arc and area of calcium were associated with significantly worse stent expansion. Fujino et al11 developed an OCT-based calcium scoring system to predict stent underexpansion: a maximum calcium angle >180°, maximum calcium thickness >0.5 mm, and calcium length >5 mm were risk factors, remarkably similar to the current study cut-offs. Thus, the current study expands our understanding of the effect of calcium on stent underexpansion from de novo coronary disease to ISR calcium (i.e., neoatherosclerotic or peri-stent calcium).
In an OCT study by Song et al15, in-stent neointimal calcium was a dominant pattern of neoatherosclerosis, observed in 60% of ISR with neoatherosclerosis, consistent with our findings and previous autopsy studies16. A small OCT study evaluating the impact of neointimal calcification on stent-in-stent ISR treatment showed a trend for a smaller stent area and diameter at the site of neointimal calcification versus proximal to the neointimal calcification17. A recent study assessed the prevalence, predictors, and implications of calcified neoatherosclerosis as the cause of ISR; ISR lesions with calcified neoatherosclerosis were associated with poorer angiographic and OCT results18.
We observed calcium fracture post re-stenting in six (14.6%) cases with good new stent expansion similar to de novo stenting19. Excimer laser coronary angioplasty or lithotripsy could disrupt calcium to facilitate full stent expansion, especially when treating an ISR lesion with a new stent20,21.
Repeat stenting of a recurrent ISR lesion is associated with chronic stent underexpansion and a high rate of adverse events22. Stent-in-stent DES treatment of ISR is associated with recurrent restenosis rates between 20% and 40%12. In another OCT study20, one third of ISR cases had multiple old stent layers associated with new stent underexpansion. A small study reported 11 recurrent ISR with two or three layers of metal after treatment using a drug-coated balloon with 13% MACE over 38 months23. Thus, although three meta-analyses have demonstrated that re-stenting should be the preferred treatment3,24,25, a drug-coated balloon should be considered when there are more than two layers of old stents unless calcium modification is used before re-stenting. However, a recent study showed that a drug-coated balloon was also less effective for ISR lesions with more than three stent layers26. Hence, multiple metallic layers should be avoided, if possible. Finally, when re-stenting an ISR lesion, it is important to optimise the ISR treatment just as it is important to optimise de novo stent implantation because new stent underexpansion is associated with a higher rate of events, as is de novo stent underexpansion.
Limitations
This was a retrospective observational study in which we included only patients with pre- and post-re-stenting OCT. Second, >50% presented beyond five years from stent implantation, which may not be representative of daily practice. Third, there were no angiographic or OCT images of the original stent implantation. Fourth, only 50% of restenotic stents were newer-generation DES.
Conclusions
When re-stenting an ISR lesion, old stent underexpansion, the amount of calcium, whether within the neointima or in peri-stent plaque, and multiple layers of old stent struts may be important determinants of new stent underexpansion which, in turn, may increase long-term events.
|
Impact on daily practice When re-stenting an ISR lesion, old stent underexpansion, the amount of coronary calcium, whether within the neointima or in peri-stent plaque, and multiple layers of old stent struts are important determinants of new stent underexpansion. New stent underexpansion is associated with adverse long-term outcome, and optimisation of ISR treatment is as important as de novo stent implantation. |
Appendix. Study collaborators
Fernando A. Sosa, MS, MBA; Abbott Vascular, Santa Clara, CA, USA. Elizabeth S. Haag, RN; St. Francis Hospital, Roslyn, NY, USA.
Acknowledgements
We would like to thank Dominic P. Francese, MPH, for assistance in preparation of this paper.
Conflict of interest statement
D. Yin reports grants from Boston Scientific during the conduct of the study. Z. Chen reports grants from Boston Scientific during the conduct of the study. A. Kirtane reports grants from Medtronic, Abbott Vascular, Boston Scientific, Abiomed, CathWorks, Siemens, Philips, ReCor Medical, and Spectranetics, outside the submitted work. M. Parikh reports personal fees from Abbott Vascular, personal fees from Medtronic, Boston Scientific, CDI, and Corsica, outside the submitted work. A. Jeremias reports a grant and personal fees from Abbott, outside the submitted work. F. Sosa is an employee of Abbott Vascular. Z. Ali reports grants and personal fees from Abbott, Medtronic, and Cardiovascular Systems Inc., other from Shockwave, and personal fees from Boston Scientific and Cardinal Health, outside the submitted work. A. Maehara reports institutional grants from Boston Scientific and Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

