Abstract
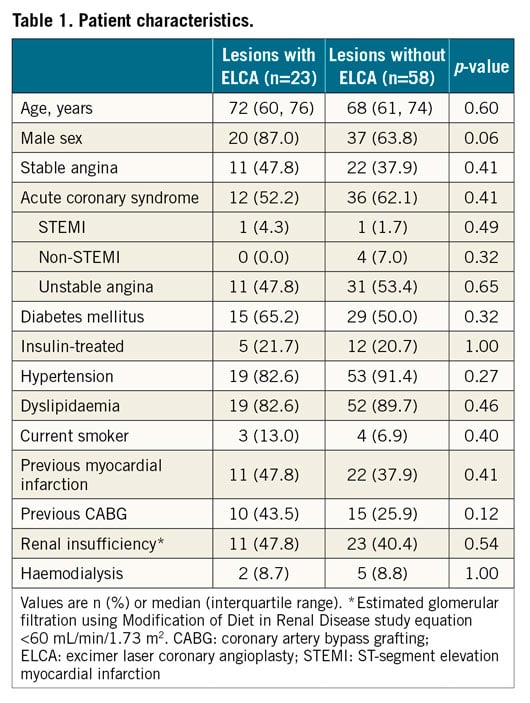
Aims: We aimed to evaluate the effectiveness of excimer laser coronary angioplasty (ELCA) to treat in-stent restenosis (ISR) due to peri-stent calcium-related stent underexpansion as assessed by optical coherence tomography (OCT).
Methods and results: We studied 81 patients (81 lesions with ISR, stent underexpansion, and peri-stent calcium >90°) who underwent OCT imaging both pre and post percutaneous coronary intervention and compared lesions treated with ELCA (n=23) vs. without ELCA (n=58). ELCA use was associated with more calcium fracture (ELCA: 61%, non-ELCA: 12%, p<0.01), larger final minimum lumen area (ELCA: 4.76 mm2 [3.25, 5.57], non-ELCA: 3.46 mm2 [2.80, 4.13], p<0.01), and a larger previously implanted stent area (ELCA: 6.15 mm2 [4.83, 7.09], non-ELCA: 4.65 mm2 [3.84, 5.40], p<0.01). In the multivariable model, ELCA use was associated with peri-stent calcium fracture (odds ratio 46.5, 95% confidence interval: 6.8, 315.9, p<0.001) that, in turn, was associated with final larger lumen and stent dimensions. Finally, contrast injection during ELCA was associated with multiple calcium fractures and fractures even in thicker calcium.
Conclusions: ELCA is effective for treating ISR with underexpansion by disrupting peri-stent calcium, facilitating better expansion of the previously implanted stent.
Introduction
Stent underexpansion is one of the most frequent causes of stent failure, especially within one year of stent implantation1,2,3. Stent underexpansion is related to poor vessel compliance, especially in severely calcified lesions4. Although calcified lesion preparation with rotational atherectomy (RA), the orbital atherectomy system (OAS), or excimer laser coronary angioplasty (ELCA) modifies calcium to prevent stent underexpansion, these devices are underutilised.
The predominant ELCA effect is rapid conversion of water into exploding vapour bubbles, thereby modifying the plaque5. Although the use of ELCA when treating in-stent restenosis (ISR) and stent underexpansion due to peri-stent calcium has been reported in previous case series6,7,8, its efficacy and mechanism have not been well studied.
Intravascular optical coherence tomography (OCT) can penetrate and characterise calcium with great resolution (10 µm)9. We used OCT to assess the mechanism of ELCA use before conventional high-pressure ballooning when treating ISR lesions with peri-stent calcium-related stent underexpansion.
Methods
STUDY POPULATION
This was a retrospective observational study at two sites (St. Francis Hospital, Roslyn, NY; NewYork-Presbyterian Hospital, New York, NY, USA). From May 2011 to April 2017, there were 139 patients who underwent OCT imaging during percutaneous coronary intervention (PCI) of 149 ISR lesions and peri-stent calcium >90°4,10. Operators decided whether to use (1) ELCA before conventional high-pressure ballooning, (2) contrast or saline flush during ELCA, and (3) additional new stent implantation. All lesions were treated by xenon-chlorine (excimer) pulsed laser (Turbo-Elite™ catheter; Spectranetics Corporation, Colorado Springs, CO, USA). The size of the laser catheter, fluency, and repetition rate were left to the operator’s discretion.
After excluding 68 lesions, 81 ISR lesions in 81 patients were included (Figure 1). The primary analysis was a comparison of angiographic and OCT acute outcomes between lesions with versus without ELCA. A secondary subgroup comparison was performed for lesions treated with or without new stent implantation.
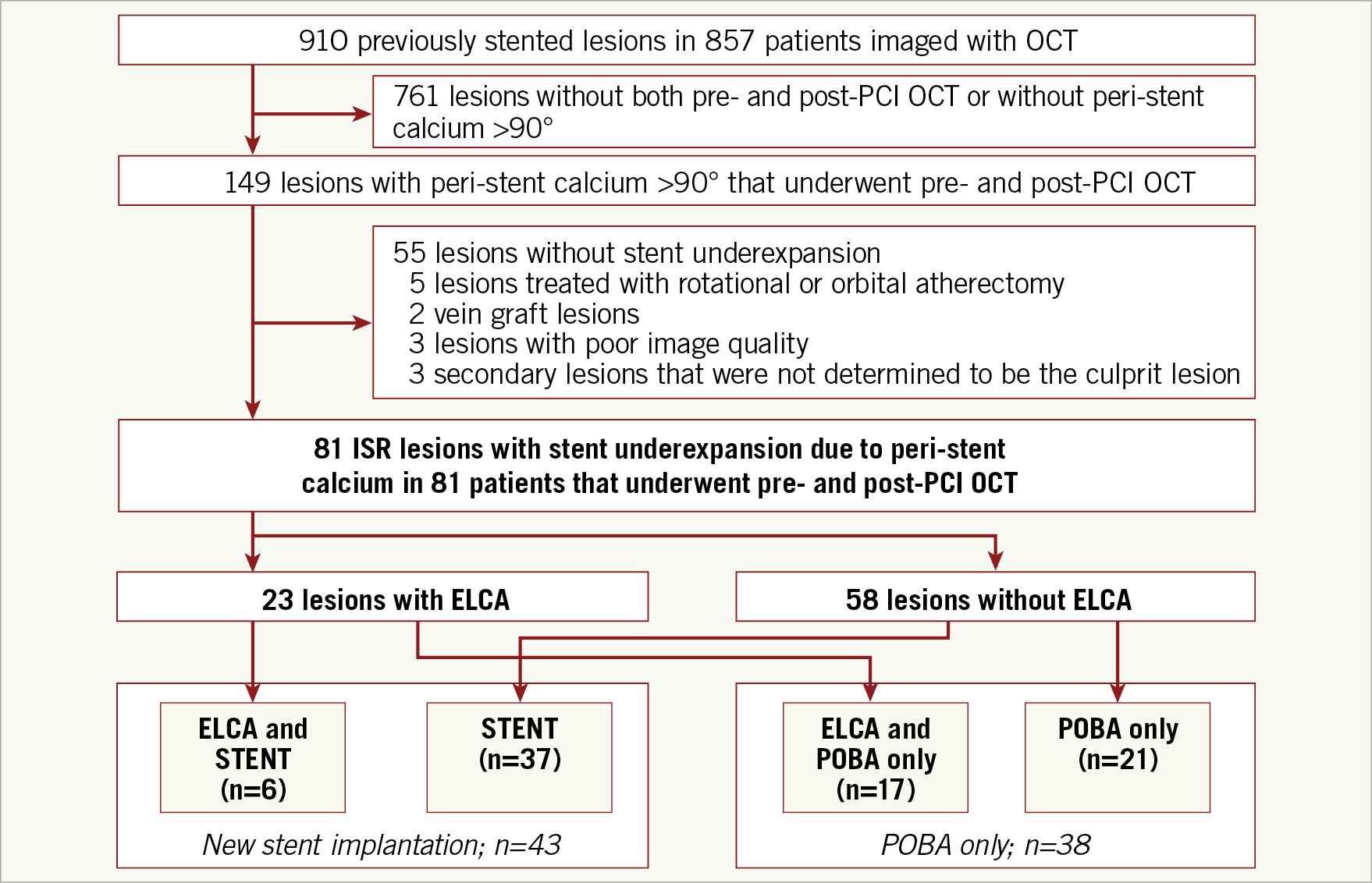
Figure 1. Study flow diagram. Overall, 81 in-stent restenosis (ISR) lesions (81 patients) with stent underexpansion due to peri-stent calcium >90° underwent pre- and post-PCI OCT and were divided into the following groups: (1) with or without excimer laser coronary angioplasty (ELCA) treatment and (2) with or without new stent implantation. POBA: plain old balloon angioplasty
CORONARY ANGIOGRAPHIC ANALYSIS
Off-line quantitative coronary angiography was performed using QAngio XA (Medis medical imaging systems, Leiden, the Netherlands) without knowledge of clinical and OCT information. The minimum lumen diameter, reference diameter, and lesion length were measured, and the restenosis pattern was classified11.
OCT IMAGE ACQUISITION AND ANALYSIS
OCT images were acquired using frequency-domain OCT: C7-XR™ or ILUMIEN™ OPTIS™ system (Abbott Vascular, Santa Clara, CA, USA) and a 2.7 Fr OCT imaging catheter (C7 Dragonfly™, Dragonfly™ Duo, or Dragonfly™ OPTIS™; Abbott Vascular). An OCT catheter was advanced distal to the lesion, and contrast media was injected at a flush rate of 3.0-4.0 mL/s through the guiding catheter with automatic pullback at a frame interval of 0.1-0.2 mm. Using previously validated criteria for OCT plaque characterisation12, off-line analysis was performed using proprietary software (Abbott Vascular) by two experienced investigators (T. Lee and A. Maehara) who were blinded to the angiographic findings and one of whom (A. Maehara) was blinded to ELCA usage.
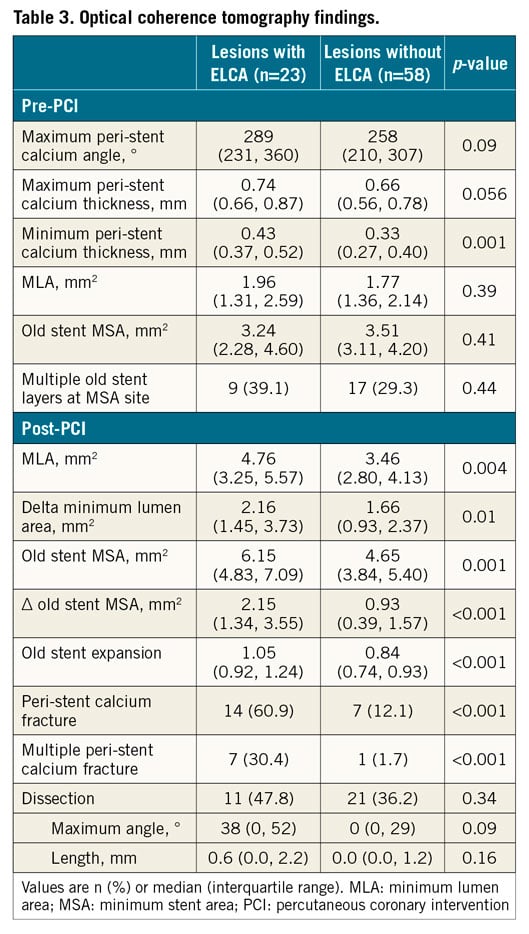
All cross-sectional OCT frames were evaluated; stent and lumen areas were measured at the minimum lumen area (MLA) site, including old stent MSA (minimum stent area) and new stent MSA (if implanted). Calcium was defined as a region with sharply delineated borders, and peri-stent calcium was defined as calcium outside and/or inside the stent (i.e., calcified neointima) (Figure 2, Figure 3, Moving image 1-Moving image 4). Calcium fracture was a slit or complete break in the calcium plate that was identified in the final OCT13; it was considered as multiple fractures when >1 calcium fracture was observed in a single frame (Figure 2, Figure 3, Moving image 1-Moving image 4),14. Fractured calcium thickness was measured at the edge of the fracture. The maximum peri-stent calcium angle was measured, and the minimum and maximum peri-stent calcium thicknesses were measured in the frame with maximum peri-stent calcium. Stent expansion was the MSA (old or new stent) divided by the average of the proximal and distal reference lumen areas; underexpansion was stent expansion <0.8 and MSA <5.0 mm21,15.

Figure 2. Representative case of ELCA treatment of in-stent restenosis. A) Pre-PCI angiogram showed severe in-stent stenosis in the distal right coronary artery (white arrow). B) Non-compliant ballooning (3.5 mm in diameter) at 22 atm showing indentation (white arrow). ELCA was then performed. C) After ELCA, non-compliant ballooning (3.75 mm in diameter) at 22 atm showing no balloon indentation. D) Final angiogram showing no residual stenosis. E) OCT before ELCA showing old stent underexpansion (stent area 3.25 mm2) due to diffuse circumferential peri-stent calcium (white arrowheads). F) Post-PCI OCT showed good expansion of the old stent (stent area 7.99 mm2) with multiple peri-stent calcium fractures (white arrows at 2, 5, and 8 o’clock). Fractured calcium thickness was 0.82 mm (inset, double-headed arrow).

Figure 3. Representative case of PCI without ELCA treatment. A) Pre-PCI angiogram showed severe in-stent stenosis in the mid left anterior descending coronary artery (white arrow). B) New stent implantation with balloon indentation (white arrow). C) Balloon dilation at 30 atm after new stent implantation. D) Final angiogram showed mild residual stenosis in the middle of the stent (white arrow). E) Pre-PCI OCT imaging showing diffuse circumferential peri-stent calcium (white arrowheads) and old stent underexpansion (stent area 4.00 mm2).
F) Post-PCI OCT imaging showing poor expansion of the newly implanted stent (stent area 4.27 mm2) without peri-stent calcium fracture.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA). Categorical data were expressed as frequencies and compared using the χ2 or Fisher’s exact test, as appropriate. Because most values were not normally distributed, continuous variables were expressed as median (interquartile range) and compared using the Mann-Whitney U test. The effect of ELCA in creating calcium fracture was tested using a multivariable logistic regression model. The effect of ELCA on acute OCT-derived outcomes (i.e., final MLA or MSA) was tested using a multivariable linear regression model after adjusting for OCT-derived morphological characteristics based on the results (Table 1-Table 3) along with known clinical risk factors4,16,17 including multiple stent layers, peri-stent maximum calcium angle, peri-stent minimum calcium thickness, pre-PCI old stent MSA, mean reference lumen area, maximum balloon pressure, scoring balloon use, and balloon/artery ratio. Qualitative and quantitative inter- and intra-observer variability was tested using kappa statistics and intra-class correlation coefficient (ICC), respectively. A p-value <0.05 was considered statistically significant.
Results
CLINICAL, ANGIOGRAPHIC, AND OCT CHARACTERISTICS
Among 81 ISR lesions, 23 were treated with ELCA and 58 were treated with just high-pressure balloon inflation (Figure 1). Mean ELCA catheter size, fluency, and repetition rate were 1.3±0.2 (0.9 mm in six lesions, 1.4 mm in 15 lesions, and 1.7 mm in two lesions), 66±9 mJ/mm2, and 64±8 Hz, respectively. Contrast flush was used during ELCA in 35% (8/23). A new stent was implanted in 53% (43/81).
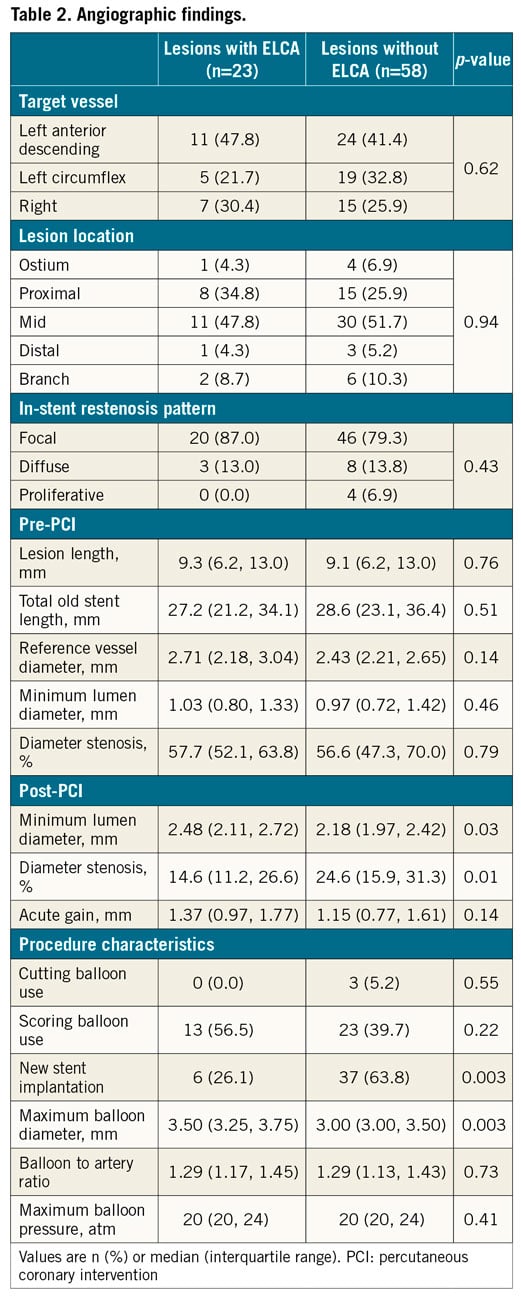
There was no significant difference in clinical characteristics between patients treated with versus without ELCA (Table 1). In the angiographic analysis (Table 2), pre-PCI findings were similar between the two groups. Post PCI, lesions treated with ELCA showed a larger angiographic minimum lumen diameter and a smaller diameter stenosis than those not treated with ELCA, even though new stent implantation was less frequent in lesions treated with ELCA (26.1%) versus those treated without ELCA (63.8%).
There was good concordance both of inter- and intra-observer agreement for the diagnosis of calcium fracture (=0.85, 0.95), maximum calcium angle (ICC=0.83, 0.91), maximum calcium thickness (ICC=0.88, 0.88), and minimum calcium thickness (ICC=0.77, 0.89), respectively. Peri-stent calcium was located only outside the stent (calcium in the native plaque) in 93% (75/81), and both inside (i.e., neointimal calcium) and outside the stent in 7% (6/81). Time from stent implantation was longer in lesions with neointimal calcium compared with only calcium outside the stent: 9.2 (8.3, 11.3) years versus 2.3 (0.8, 5.6) years, p=0.002. In the pre-PCI OCT analysis, the maximum peri-stent calcium angle and thickness measured 273° (median) and 0.70 mm (median), respectively; the old stent MSA measured 3.47 mm2 (median) without any difference between the ELCA and non-ELCA groups. Irrespective of new stent implantation, the final old stent MSA (6.15 mm2 [4.83, 7.09] versus 4.65 mm2 [3.84, 5.40], p<0.01) and the acute gain of the old stent MSA (2.15 mm2 [1.34, 3.55] versus 0.93 mm2 [0.39, 1.57], p<0.001) were larger in lesions treated with ELCA versus without ELCA along with more peri-stent calcium fracture (61% versus 12%, p<0.001) (Table 3). Among 23 lesions treated with ELCA, there were 13 lesions with pre-intervention, post-ELCA (but before post-dilation with high pressures), and final OCT imaging. Calcium fracture was observed in one lesion post ELCA, while in nine lesions (69%) calcium fracture was observed post dilation or after new stent implantation (p=0.004). Accordingly, the ΔMLA was greater from post ELCA to final (1.91±0.45 mm2) than pre-intervention to post ELCA (0.70±0.83 mm2, p=0.02). In non-calcified segments, dissections were small without difference between lesions with versus without ELCA (dissection angle, 38 versus 0°, p=0.09).
All peri-stent calcium fractures were observed in calcium outside the stent. This was consistent in six lesions with calcium both inside and outside of the stent (prevalence of peri-stent calcium fracture: 2/2 with ELCA versus 0/4 without ELCA, p=0.07) or in 75 lesions with calcium only outside the stent (12/21=57% with ELCA versus 7/54=13% without ELCA, p<0.001).
IMPACT OF CONTRAST INJECTION DURING ELCA
Irrespective of new stent implantation, 35% (8/23) of ELCA procedures used contrast flush, and 65% (15/23) used saline flush. ELCA procedures performed with contrast flush more often had multiple calcium fractures (63% versus 13%, p=0.03), even though lesions treated with contrast flush had greater peri-stent calcium (and thicker calcium) versus lesions treated using saline flush (360° versus 266°, p=0.01). Fractured calcium was thicker in lesions treated by ELCA with contrast (0.73 mm) versus ELCA without contrast (0.45 mm) or without ELCA (0.32 mm), but not between lesions treated by ELCA with versus without contrast (Figure 4).
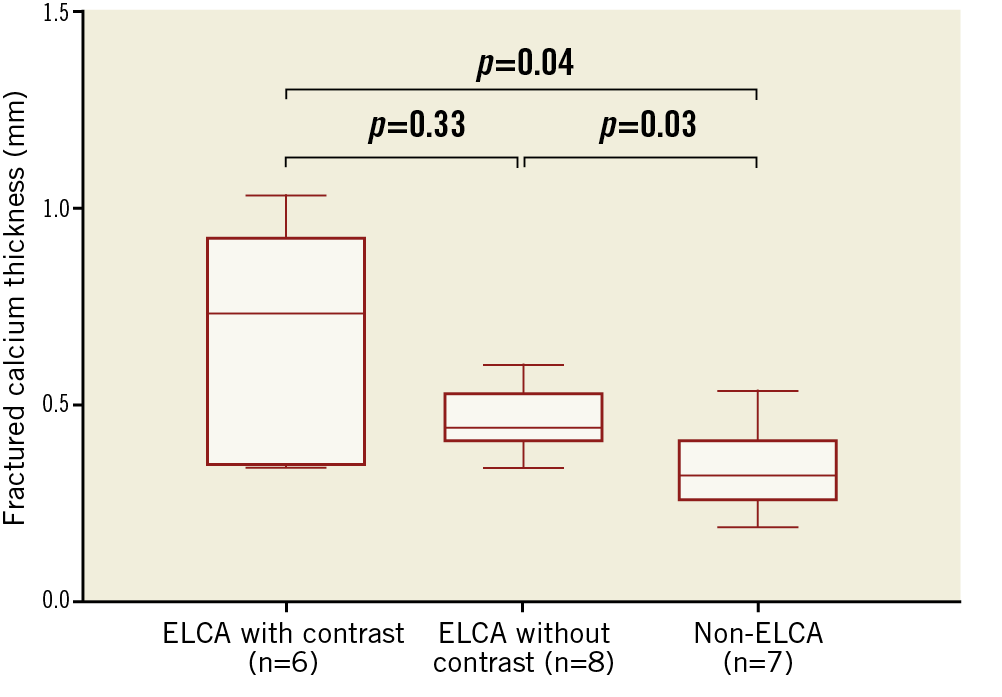
Figure 4. Calcium fracture thickness among lesions without ELCA, ELCA without contrast, and ELCA with contrast. In lesions treated using ELCA with contrast, fractured calcium was thicker than in the other two groups.
IMPACT OF NEW STENT VERSUS BALLOON DILATION ALONE
We performed subgroup comparison between lesions treated with versus without ELCA in lesions with or without new stent implantation (i.e., balloon only), separately (Table 4). The effect of ELCA (more peri-stent calcium fractures resulting in a greater increase in the old stent MSA and a larger final MLA) was consistent in both subgroups with a greater difference in lesions treated with a new stent versus balloon only.

CLINICAL AND OCT CHARACTERISTICS ASSOCIATED WITH CALCIUM FRACTURE AND IMPROVED OLD STENT EXPANSION
In a multivariable logistic regression analysis to predict peri-stent calcium fracture (seen in 26%, 21/81), ELCA (odds ratio [OR] 46.5, 95% confidence interval [CI]: 6.8, 315.9, p<0.001), maximum peri-stent calcium angle per quadrant (OR 5.1, 95% CI: 1.46, 17.9, p=0.01), and minimum peri-stent calcium thickness per 0.1 mm (OR 0.99, 95% CI: 0.98, 0.99, p=0.01) were independently associated with peri-stent calcium fracture. Because there were only 21 calcium fractures, only three covariates were entered into the model.
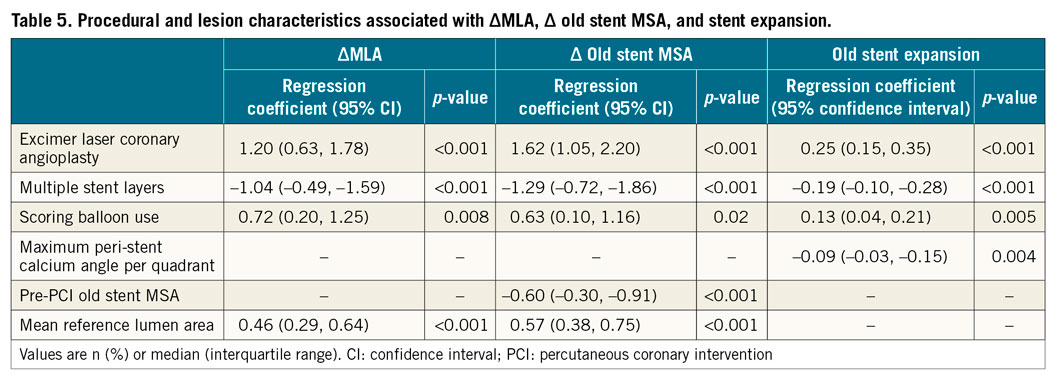
In a multivariable linear regression model to predict (1) MLA increase, (2) old stent MSA increase, and (3) post-PCI old stent expansion, ELCA and scoring balloon use were significantly associated with all three endpoints, whereas multiple stent layers of old stents and maximum peri-stent calcium angle were associated with lesser stent expansion (Table 5).
There was one coronary perforation and one coronary dissection requiring additional stent implantation in patients treated without ELCA, one dissection requiring additional stent implantation in a patient treated with ELCA without contrast, and no coronary perforation or dissection requiring additional stent implantation in patients treated with ELCA with contrast flush.
Discussion
OCT provided insights into the mechanism of the efficacy of ELCA for ISR lesions due to OCT-documented peri-stent calcium. The major findings were as follows: (1) ELCA created more calcium fractures even if the calcium was thicker, and this effect was more pronounced with contrast flush; (2) ELCA resulted in larger final lumen dimensions and better stent expansion compared with high-pressure balloon dilation alone.
ELCA was introduced two decades ago to treat complex coronary artery disease, including ISR and calcified lesions. Even in the drug-eluting stent (DES) era, stent underexpansion remains an important risk factor for stent thrombosis and restenosis3, and management of ISR lesions with an underexpanded stent due to severe peri-stent calcium remains a technical challenge. Although treatment of stent underexpansion due to severe calcium is a niche application for ELCA, alternatives for these patients are limited and include coronary artery bypass graft surgery, RA, or OAS, all with their own potential problems (burr entrapment, distal embolisation of microparticles, and vessel damage). There is limited published clinical experience18,19.
Mintz et al20 reported that ELCA in non-stented lesions increased lumen area by both atheroablation and vessel expansion without a reduction in lesion calcium, and superficial fibrocalcific deposits developed a fragmented appearance when imaged by intravascular ultrasound (IVUS). Another IVUS study showed that lumen enlargement from ELCA during the treatment of ISR was the result of neointimal tissue ablation21. Even though the efficacy of ELCA to treat an underexpanded restenotic stent has been reported7, the precise mechanisms have not been well understood. Because IVUS is unable to penetrate calcium with a limited resolution compared with OCT, it is difficult to assess the efficacy of ELCA treatment using IVUS, especially when treating ISR lesions. We demonstrated that ELCA-treated lesions had more calcium fracture, especially in more severely calcified lesions with greater calcium thickness where calcium fracture was necessary to correct stent underexpansion. This current study also highlighted the value of contrast flush during ELCA, including multiple calcium fractures even in the setting of more severe peri-stent calcium. Blood and radiographic contrast absorb 308 nm light avidly, and the presence of either of these two liquids exacerbates vapour bubble explosion while preventing light from reaching the tissue22. While ELCA with saline flush is recommended to reduce complications such as coronary dissection and perforation due to high-pressure waves, in cases of underexpanded stents where the stents are protective, contrast medium flush could optimise the ELCA effect6,7,8. Contrast injection during ELCA should be reserved for thick, extensive, and resistant peri-stent calcium5,6.
In two studies, chronic stent underexpansion persisted after high-pressure balloon inflation and a third stent implantation for recurrent ISR23,24. In line with these studies, the current analysis suggested that multiple layers of underexpanded stents not only triggered recurrent stent failure, but also constituted a challenging substrate for reintervention. Therefore, it is important to correct stent underexpansion even when treating the first ISR event, especially when implanting a second stent and before the problem becomes magnified during a second or third ISR episode.
Case reports have indicated the effectiveness of RA for lesions with non-dilatable calcified neointima25. One IVUS study reported that calcified neointima was associated with increased calcium outside the stent26. ELCA treatment may be effective to treat undilatable calcified neointima because these lesions also typically have heavy calcium outside the stent struts.
In the present study, approximately 60% of ISR lesions showed stent underexpansion as a main cause of stent restenosis. Contrary to the bare metal stent era, stent underexpansion appeared to be the main cause of DES restenosis because of suppressed neointimal hyperplasia compared with bare metal stents27. IVUS-guided PCI can minimise stent underexpansion28. The importance of stent underexpansion has been substantiated in OCT studies2.
Limitations
This was a retrospective observational study. The number of patients was relatively small, precluding subgroup analysis, especially the number of lesions treated with ELCA, probably causing selection bias.
Conclusions
ELCA is effective for treating ISR with underexpansion due to peri-stent calcium. OCT clarifies the mechanism showing that ELCA disrupts peri-stent calcium facilitating better expansion of the previously implanted stent.
|
Impact on daily practice ELCA use to treat ISR due to peri-stent calcium-related stent underexpansion was associated with better acute outcomes (larger lumen and stent area). Lesions treated using ELCA had more fracture of peri-stent calcium detected by OCT, especially using ELCA with contrast flush. |
Acknowledgements
The authors thank Dong Yin, MD, Fernando A. Sosa, MS, MBA, and Dominic P. Francese, MPH, for their help in this study.
Conflict of interest statement
G. Mintz declares receiving honoraria from Boston Scientific/Philips. A. Maehara declares receiving grant support from Boston Scientific and Abbott Vascular. Z. Ali declares receiving grant support from Cardiovascular Systems Inc, Abbott Vascular, and ACIST Medical, and being a consultant to Boston Scientific, Abbott Vascular, and ACIST Medical. A. Jeremias declares being a consultant to and receiving honoraria from Boston Scientific. L. Song declares receiving a research grant from Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Pre-PCI OCT. ELCA treatment of in-stent restenosis (same lesion as Figure2). OCT before ELCA showing old stent underexpansion due to diffuse circumferential peri-stent calcium.
Moving image 2. Post-PCI OCT. ELCA treatment of in-stent restenosis (same lesion as Figure2). Post-PCI OCT showed good expansion of the old stent with multiple peri-stent calcium fractures.
Moving image 3. Pre-PCI OCT. PCI without ELCA treatment (same lesion as Figure3). Pre-PCI OCT imaging showing diffuse circumferential peri-stent calcium and old stent underexpansion.
Moving image 4. Post-PCI OCT. PCI without ELCA treatment (same lesion as Figure3). Post-PCI OCT imaging showing poor expansion of the newly implanted stent without peri-stent calcium fracture.

