Abstract
Aims: Optical coherence tomography (OCT) can delineate calcified plaque without artefacts. The aim of this study was to evaluate the ability of OCT to quantify calcified plaque in ex vivo human coronary arteries.
Methods and results: Ninety-one coronary segments from 33 consecutive human cadavers were examined. By intravascular ultrasound (IVUS), 32 superficial calcified plaques, defined as the leading edge of the acoustic shadowing appears within the most shallow 50% of the plaque plus media thickness, were selected and compared with corresponding OCT and histological examinations. The area of calcification was measured by planimetry. IVUS significantly underestimated the area of calcification compared with histological examination (y=0.39x+0.14, r=0.78, p<0.001). Although OCT slightly underestimated the area of calcification (y=0.67x+0.53, r=0.84, p<0.001), it showed a better correlation with histological examination than IVUS.
Conclusions: Both OCT and IVUS underestimated the area of calcification, but OCT estimates of the area of calcification were more accurate than those estimated by IVUS. Thus, OCT may be a more useful clinical tool to quantify calcified plaque.
Introduction
Intravascular ultrasound (IVUS) is widely used for characterising coronary plaques, and can detect calcification with a high sensitivity and specificity as compared with angiography.1 However, it’s difficult to evaluate the degree of calcification quantitatively via IVUS because ultrasound can not penetrate the calcification. Optical coherence tomography (OCT) has recently been developed as a high-resolution imaging modality, providing an accurate evaluation of coronary plaque morphology.2-4 Furthermore, OCT can delineate calcified plaque without acoustic shadowing. Therefore, we hypothesised that OCT would provide accurate quantification of the calcified plaque. To test this hypothesis, we conducted an ex vivo validation study using OCT.
Methods
Study population
We examined 91 coronary arterial segments from 33 consecutive human cadavers (23 male and 10 female) with both OCT and IVUS imaging. Four of these 33 cadavers were diagnosed with coronary heart disease as the cause of death (12%). The study protocol was approved by the ethics committee of Kawasaki Medical School, and written informed consent was obtained from each family. Segments measuring about 5 cm in length were obtained beginning at the ostial site of the three major coronary arteries and harvested at autopsy within three hours of death. The surrounding soft tissues were dissected from each specimen. After flushing the vessel with saline, small arterial perforators and the branches were tied off with sutures, and the distal end of the artery was occluded with a large cork. A 7 Fr sheath was sewn into the proximal end of the artery to complete the closed system. Saline (0.9%), which was kept at a temperature of 37°C, was infused through the side arm of the sheath to create a blood-free environment. The pressure inside the coronary artery was maintained at a physiologic level (60 to 80 mmHg) with a sphygmomanometer connected to the infusion.
Intracoronary imaging
An intravascular OCT catheter (ImageWire®, model M2 Cardiology Interface System; LightLab Imaging, Westford, MA, USA) and an IVUS catheter (Atlantis SR Pro® 2.5 Fr, 40-MHz; Boston Scientific, Natick, MA, USA) were inserted sequentially through the diaphragm of the sheath. Serial images of OCT and IVUS were obtained using respective automatic pullback devices at a rate of 1.0 mm/s. The OCT images were processed and analysed using proprietary software from LightLab Imaging. IVUS images were analysed off-line by a commercially available image processing software (Netra 3D IVUS system; ScImage, Los Altos, CA, USA). For analysis of the IVUS images, external elastic membrane (EEM), lumen, and plaque plus media cross-sectional area (CSA) were measured. Plaque burden was calculated as (plaque plus media CSA/EEM CSA) x 100 (%). In the analysis of the IVUS images, calcification was defined as bright echoes with acoustic shadowing. IVUS can visualise only the leading edge of calcification because high frequency ultrasound does not penetrate the calcification.5 Therefore, we measured the area of the dense bright lesions in the IVUS images as the calcification area. The arc of calcification was measured by using an electronic protractor centred on the lumen.
In the analysis of the OCT images, calcification was defined as well-delineated, signal-poor regions with sharp borders.2,3 The limited depth of penetration (approximately 2.0 mm) may restrict the ability of OCT to visualise the entire superficial calcification circumference. The area of calcification that was clearly imaged by OCT was measured up to OCT signal drop off. The arc of calcification and lumen CSA was also measured by OCT.
Histological examination
After OCT and IVUS pullback imaging, each coronary artery was pressure-fixed in 10% neutral buffered formalin. Following fixation for 48 hours, standard paraffin embedding was performed. In every 400 µm of the coronary arteries, two series of 4 µm thickness sections were cut and stained with haematoxylin and eosin and elastica van Gieson techniques. Histological images were digitised by computer to evaluate the same measurements as previously described.6 The arc and area of calcification was evaluated.
Identification of superficial calcified plaque
Thirty-two superficial calcified plaques, defined by the leading edge of the calcification appears within the inner-most half of the plaque plus media thickness in the analysed single cross-section, were identified in IVUS images and compared with corresponding OCT and histological examinations. Assessments of OCT and histological images were done at the same sites identified as superficial calcified plaque in the IVUS image; located according to the distance from anatomical landmarks such as side branches.
Statistics
Continuous variables are presented as mean values ±SD when indicated. ANOVA with post hoc comparisons using the Scheffé procedure and t tests were used to assess the significance of the results in this study. The intra- and inter-observer agreement was assessed by determining the mean and SD of the between-observation and between-observer differences, respectively. A p value of <0.05 indicated statistical significance.
Results
OCT, IVUS, and histological characteristics of superficial calcified lesion are shown in Table 1.
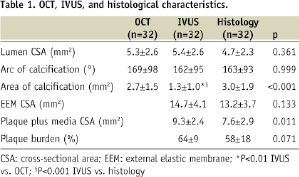
Both OCT and IVUS evaluations of arc of calcification were similar to those of the histological examination. There was a better one-to-one correspondence in areas of calcification between the OCT and the histological examination than between the IVUS and the histological examination (Figures 1 and 2).
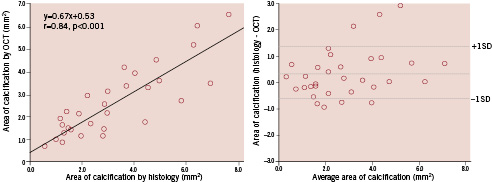
Figure 1. Comparison of area of calcification evaluated by optical coherence tomography (OCT) vs. histological examination (left); and Bland–Altman test for OCT vs. histological examination in measurement of area of calcification (right). There was a good one-to-one correspondence in areas of calcification between OCT and the histological examination.
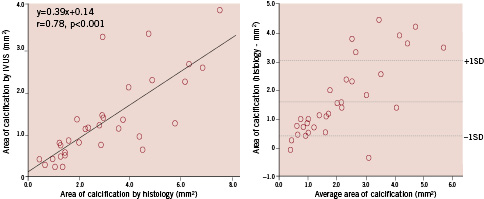
Figure 2. Comparison of area of calcification evaluated by intravascular ultrasound (IVUS) vs. histological examination (left); and Bland–Altman test for IVUS vs. histological examination in measurement of area of calcification (right). There was a better one-to-one correspondence in areas of calcification between OCT and histological examination than between IVUS and histological examination.
Reproducibility of measurements of calcification areas assessed by OCT was good (intra-observer differences, –0.10±0.59 mm2, and inter-observer differences, 0.16±0.60 mm2). Correlation coefficients were high for repeated measurements by the same observer (r=0.92) and for measurements by two different observers (r=0.91). Representative images of superficial calcified lesion are shown in Figure 3.
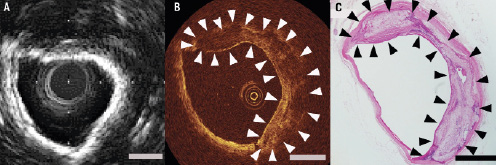
Figure 3. Representative images of superficial calcified lesion by intravascular ultrasound (IVUS), optical coherence tomography (OCT) and histological examination. Histological examination revealed severe calcification (Panel C, haematoxylin and eosin stain). An IVUS image of calcified lesion showed the bright reflection and frequent saturation artefact from the inner-most surface of the calcium (Panel B). Therefore, it’s difficult to evaluate the degree of calcification quantitatively via IVUS. Although penetration of the OCT signal was limited, OCT could visualise the calcification without any artefacts. In the OCT image, calcification was characterised by well-delineated, signal-poor region with sharp border (Panel A). Calcification (arrow). Scale bar, 1 mm.
Discussion
Previous studies have shown that IVUS can detect coronary lesion calcification more frequently and reliably than by fluoroscopy.1 Although target lesion calcification may be a possible marker of the lesion compliance, a recent study failed to predict stent expansion by IVUS, derived extent and degree of calcification.6 This may be due to the fact that IVUS can only visualise the surface of the calcium. In fact, plaque component behind a superficial calcification could not be detected and quantitated by IVUS.7 On the other hand, OCT can delineate calcified plaque without acoustic shadowing3. The current study indicated that superficial calcification could be quantified more accurately by using OCT rather than IVUS. Thus, the area of calcification assessed by OCT might be related to stent expansion.
Stent underexpansion is the main factor associated with restenosis and stent thrombosis after implantation of bare-metal and drug-eluting stents.8-13 Cheneau et al reported the results of a systematic IVUS analysis performed in a large population of patients undergoing de novo stenting and showed that stent underexpansion was the most frequent finding in sub-acute stent thrombosis after bare-metal stent implantation.14 In the drug-eluting stent era, final stent expansion is still one of the strongest predictor of restenosis. Hong et al reported that the restenosis rate was highest in lesion with stent area <5.5 mm2 after sirolimus-eluting stent implantation15. Furthermore, Okabe et al reported that lesions causing stent thrombosis after drug-eluting stent implantation have small minimum stent areas16. Therefore, prediction and prevention of stent underexpansion should be considered important before and following the stent implantation procedure. If the area of calcification assessed by OCT can predict stent expansion, prevention of stent underexpansion might be possible by aggressive pre-dilatation using either balloon angioplasty or rotational atherectomy prior to stent deployment. Furthermore, because the calcification can be delineated by OCT, accurate sizing of the rotational atherectomy may be possible. Although, in our preliminary observations, tissue sample was limited, OCT might be a useful clinical tool for guiding a percutaneous coronary intervention strategy and improve the outcome of patient with coronary artery disease after implantation of drug-eluting stent by preventing restenosis and stent thrombosis.
For an accurate measurement of the entire calcification area, or imaging of deep vascular structures, the limited depth of penetration of OCT could pose a problem. Even in lesions with superficial calcification, accurate measurements of the entire calcification area assessed by OCT might be difficult due to the limited depth of penetration. Indeed, OCT underestimated the area of calcification compared with histological examination in this study. We previously reported that OCT assessments of lipid tissue with thin fibrous cap (the thickness of the fibrous cap smaller than 200 um) were achieved with 92% sensitivity and 75% specificity while overall OCT assessments of lipid tissue were achieved with 77% sensitivity and 75% specificity.17 These data suggested that the small penetration of OCT may limit the assessment of the entire plaque morphology especially in lesion with thick fibrous cap. Improved penetration of the OCT catheter would be necessary for measuring the exact area of calcification.
Limitations
OCT and IVUS images were obtained by using automatic pullback devices. Using landmarks such as side branches, we were able to compare similar points. However, the exact point of comparison between the histological images and OCT/IVUS images was very difficult. Some differences of sampling location may influence the arc of calcification and/or area of calcification results.
Ex vivo conditions are obviously different as compared with in vivo conditions and careful interpretation is still required. In vivo clinical conditions, proximal balloon occlusion and continuous saline flushing are necessary to establish a blood-free environment for OCT light penetration to the vessel wall in the M2 OCT system. Therefore, in some cases, the residual scattered blood degrades the OCT image quality and measurements by OCT may be smaller than those by IVUS in vivo.18 Measurement discrepancies might be related to the decrease in intracoronary pressure during OCT imaging resulting from proximal balloon occlusion. On the other hand, in this ex vivo study, OCT images were obtained in a saline solution (completely blood-free environment) without the need of proximal balloon occlusion. The quality of the OCT image may be better than that in the in vivo OCT study and discrepancies in measurements between OCT and IVUS might not be significant in this ex vivo study.
Conclusion
Both OCT and IVUS underestimated the area of calcification, but OCT estimates of the area of calcification were more accurate than those estimated by IVUS. Thus, OCT may be a more useful clinical tool to quantify calcified plaque.

