
Abstract
Aims: The role of intraprocedural optical coherence tomography (OCT) on the long-term clinical outcome of percutaneous coronary interventions (PCI) remains undefined. The aim of the present study was to evaluate the impact of quantitative OCT-defined suboptimal stent implantation at long-term follow-up.
Methods and results: In the context of the multicentre Centro per la Lotta contro l’Infarto – Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) registry, we compared the long-term PCI outcome of 1,211 patients from 13 independent OCT-experienced centres according to end-procedural OCT findings. OCT assessment revealed suboptimal stent implantation in 30.9% of lesions, with an increased prevalence in patients experiencing device-oriented cardiovascular events (DoCE) (52.8% vs. 28.0%, p<0.001). At a median follow-up of 833 (interquartile range 415-1,447) days, in-stent minimum lumen area (MLA) <4.5 mm2 (HR 1.82, p<0.001), distal stent edge dissection >200 µm (HR 2.03, p=0.004), and significant reference vessel plaque and lumen area <4.5 mm2 at either the distal (HR 5.22, p<0.001) or proximal (HR 5.67, p<0.001) stent edges were independent predictors of device failure. Conversely, in-stent MLA/mean reference lumen area <70%, acute stent malapposition, and intra-stent plaque/thrombus protrusion were not associated with worse outcomes. Using multivariable Cox hazard analysis, the presence of at least one of the significant criteria for suboptimal OCT stent deployment was confirmed as an independent predictor of DoCE (HR 1.92, p=0.001).
Conclusions: Suboptimal stent deployment, defined according to specific quantitative OCT criteria, was confirmed as an independent outcome predictor at long-term follow-up.
Abbreviations
IVUS: intravascular ultrasound
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
DoCE: device-oriented cardiovascular events
MLA: minimum lumen area
SM: stent malapposition
Introduction
The role of intravascular imaging guidance (intravascular ultrasound [IVUS] and optical coherence tomography [OCT]) to optimise percutaneous coronary interventions (PCI) is still much debated1-4. IVUS studies have shown that the two strongest and most consistent predictors of early stent thrombosis or restenosis after bare metal stent (BMS) or drug-eluting stent (DES) implantation are stent underexpansion (or a smaller intra-stent minimum lumen area [MLA] as a consequence of tissue/thrombus protrusion after stenting a culprit lesion in a patient presenting with an acute coronary syndrome [ACS]) and geographic miss (including uncovered edge dissections)5. These IVUS predictors of device-oriented cardiovascular events (DoCE) have been confirmed by OCT studies4,6,7.
In this context, the Centro per la Lotta Contro l’Infarto – Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) project was specifically designed to translate OCT findings into an effective clinical improvement. However, one limitation of previous IVUS and OCT reports – including the CLI-OPCI II study – resided in the relatively short period of observation and the limited number of adverse events, leaving some uncertainties on the long-term clinical role of the metrics that were identified.
The aim of the present CLI-OPCI substudy (CLI-OPCI LATE) was to evaluate the impact of quantitative OCT-defined suboptimal stent implantation at long-term follow-up. In particular, we explored the predictive accuracy of the already validated criteria of suboptimal stent deployment in a large study population including 1,211 patients with a median follow-up duration of approximately three years.
Methods
STUDY DESIGN AND ENDPOINTS
The design and aims of the CLI-OPCI project have been described previously7,8. Briefly, the CLI-OPCI registry, aiming to assess the impact of OCT findings during PCI, was collected from participating centres (Appendix) who submitted data on all consecutive PCIs performed with the support of OCT imaging. Due to the exploratory nature of the project, only end-procedural OCT images were collected and included in the final analyses; indication for OCT, PCI technique, and additional manoeuvres derived from OCT use were left to each operator’s choice. By protocol, all included cases had at least one good quality OCT pullback in the treated vessel that was carried out at the end of the procedure with a sufficient acquisition length to address the whole stented segment(s) and at least 5 mm of the adjacent reference segment(s)1,9,10, with the exclusion of ostial lesions, in which proximal references were not assessed.
The correlation between end-procedural OCT-defined suboptimal stent deployment and long-term DoCE constituted the primary endpoint of this substudy; the impact of individual predictive quantitative OCT criteria previously validated was also evaluated. DoCE were a composite of cardiac mortality, target vessel myocardial infarction (MI; defined as CK-MB >3 times the upper limit of normal), and target lesion revascularisation (TLR)11. Outcomes were defined according to the Academic Research Consortium guidelines12 and adjudicated blindly by a clinical events committee. The project was approved by the local ethics board and all patients provided written informed consent for the index procedure, phone/direct visit follow-up, and anonymous data management. This work was supported by the Centro per la Lotta Contro l’Infarto – Fondazione Onlus (Rome, Italy) and the authors were solely responsible for the design, conduct, and final content of this study.
PROCEDURES AND IMAGING ACQUISITION
Coronary angiography and PCIs were performed using standard techniques and catheters, according to the local common practice. OCT images was acquired by means of the C7-XR™ FD-OCT™ Imaging System or the OPTIS™ Imaging System (both St. Jude Medical, St. Paul, MN, USA) with a non-occlusive technique according to a well-standardised methodology1,9,10. Treatment choices (i.e., interventional technique and stent selection) were left to each operator’s discretion, post-procedural dual antiplatelet therapy was recommended for at least twelve months, and patients were regularly followed by means of periodic phone calls and/or direct visit. In case of any adverse event or hospitalisation during the follow-up, additional visits were planned to obtain source documents and for proper adjudication of events.
OCT ANALYSES AND DEFINITIONS
All angiographic and OCT images were analysed in a blinded fashion off-line by expert readers at a central core laboratory (Rome Heart Research). OCT images were reconstructed using the Offline Review Workstation ILUMIEN™/OPTIS™ system (St. Jude Medical)1, while dedicated automatic edge-detection software (Medis medical imaging systems bv, Leiden, the Netherlands) was adopted for quantitative coronary angiographic (QCA) assessment13. Definitions for OCT were derived from available consensus documents1, and quantitative cut-offs were derived from the CLI-OPCI registries6,7 using the receiver operating characteristic (ROC) curve and highest Youden’s index (J) to confirm the predictive accuracy and the maximum potential effectiveness14,15:
– Malapposition: stent strut detachment from adjacent vessel wall >200 μm16,17;
– Edge dissection: linear rim of tissue ≥200 μm in width and with a clear separation from the vessel wall or underlying plaque adjacent (<5 mm) to a stent edge1,17;
– Reference lumen narrowing: lumen area <4.5 mm2 in the presence of significant (>70%) residual plaque adjacent to a stent edge7,17;
– In-stent minimum lumen area (MLA): MLA <4.5 mm2 assessed along the entire stent length7,17;
– Residual stenosis: in-stent MLA <70% of the average reference lumen area7,17;
– Eccentricity index: maximum stent diameter/minimum stent diameter ratio <0.7 at MLA17;
– Intra-stent plaque/thrombus protrusion: tissue prolapse ≥500 μm in thickness among stent struts into the vessel lumen16,18.
Suboptimal OCT stent deployment required the presence of at least one of these OCT findings significantly associated with DoCE.
STATISTICAL ANALYSIS
Mean (±standard deviation) or median (1st-3rd quartile) was used to describe continuous variables in case of normal or skewed distribution, respectively; percentages were used to report discrete variables. The Student’s t-test, Mann-Whitney U test, χ² test, and Fisher’s exact test were applied for bivariate analyses when appropriate. DoCE were evaluated on a per-patient hierarchical basis, compared with the log-rank test, and summarised as Kaplan-Meier estimates. A landmark analysis at one year was also performed to provide separate descriptions of the midterm and late events. TLR and stent thrombosis were analysed on both a per-patient and a per-lesion basis, and a generalised mixed model analysis was performed to exclude differences due to lesion and patient level clustering. All variables reported in Table 1, Table 2 and Table 3 were tested for bivariate association with DoCE and, if nominally significant (p<0.05), were simultaneously forced into a Cox regression model to identify independent outcome predictors and to calculate their adjusted hazard ratios (HRs). The final Cox regression model included the following variables: age, left ventricular ejection fraction (LVEF), diabetes mellitus, chronic kidney disease, family history of coronary artery disease (CAD), prior MI, angiographically ambiguous lesion (i.e., intermediate lesion with irregular contour and/or haziness), ostial lesion treatment, bare metal stent (BMS) or bioabsorbable vascular scaffold (BVS) usage, and suboptimal final OCT result. Statistical analyses were carried out using SPSS Predictive Analytics Software (PASW), Version 22.0 (IBM Corp., Armonk, NY, USA) and adopting a two-tailed p-value <0.05 for statistical significance.
Results
Between 2009 and 2013, 1,211 patients with 1,422 lesions undergoing end-procedural OCT assessment were enrolled in this registry from 13 independent OCT-experienced centres. Table 1 and Table 2 summarise the clinical and procedural characteristics of the study population. Median patient age was 64 (interquartile range [IQR] 56-72) years, with 20.8% females. The prevalence of diabetes mellitus and chronic kidney disease was 21.6% and 13.6%, respectively. A history of previous MI was present in 21.4% of cases, previous coronary revascularisation in 30.9%, and about half of the patients had multivessel disease. An ACS was the admission diagnosis in 58.2% of patients, including acute MI in 41.6%.
Treated lesions usually had a complex profile (Ellis Class B2/C, 78.2%), predilation was performed in 73.0% of lesions, high-pressure stent post-dilatation in 55.8%, DES implantation in 73.1%, and multiple overlapping stents in 21.1%. A satisfactory angiographic result (residual stenosis <30% with TIMI 3 flow) was obtained in 97.7% of treated lesions with 2.9% periprocedural MI.
End-procedural OCT assessment disclosed an in-stent MLA <4.5 mm2 in 23.8% with asymmetry in 6.4% of the stented lesions, stent underexpansion in 22.4%, edge dissection in 12.0%, acute residual stent malapposition (SM) in 52.3%, intra-stent plaque/thrombus protrusion in 27.5%, and reference lumen narrowing in 6.3%.
During the observed median follow-up of 833 (IQR 415-1,447) days, 11.9% of the patients experienced a DoCE including 3.4% cardiac mortality, 4.3% non-fatal target vessel MI, and 8.6% TLR (Table 4). Excluding periprocedural MI, mean time-to-DoCE was 754 (IQR range 376-1,383) days, with 55.6% of adverse events occurring within the first twelve months after the procedure and 44.4% occurring after twelve months. The rate of patients lost after the first follow-up contact (usually 30-180 days after PCI) was 6.8%. Only one case of premature dual antiplatelet therapy (DAPT) discontinuation was documented in these cases. DAPT regimens included clopidogrel in 68.7%, prasugrel in 19.2%, and ticagrelor in 12.1% of patients, with no significant difference between patients with vs. those without adverse events.
CLINICAL PREDICTORS
When compared to patients with event-free survival, patients experiencing DoCE during follow-up showed a higher baseline risk profile including older age (66 vs. 64 years, p=0.020), lower median LVEF (54% vs. 55%, p=0.003), a higher prevalence of diabetes mellitus (34.0% vs. 20.0%, p<0.001) and chronic kidney disease (20.1% vs. 12.7%, p=0.011), and a more frequent history of prior MI (31.3% vs. 20.1%, p=0.003) (Table 1).
Regarding procedural characteristics, patients with DoCE were characterised by higher BMS use (27.0% vs. 14.8%, p<0.001) and more frequent treatment of an ostial lesion (10.1% vs. 6.0%, p=0.046), or an angiographically ambiguous lesion (14.6% vs. 9.2%, p=0.017) (Table 2).
In lesions associated with any adverse event during follow-up, OCT analyses revealed a significantly higher prevalence of suboptimal stent deployment in terms of in-stent MLA <4.5 mm2 (35.4% vs. 22.1%, p<0.001), dissection >200 µm at the distal stent edge (10.7% vs. 5.6%, p=0.007), and reference lumen area <4.5 mm2 in the presence of residual significant plaque at either the distal (15.7% vs. 3.1%, p<0.001) or proximal (8.4% vs. 1.1%, p<0.001) stent edge (Figure 1). Conversely, other parameters such as eccentric stent expansion at the MLA site (12.4% vs. 10.3%, p=0.479), in-stent MLA <70% of the average reference lumen area (25.8% vs. 21.9%, p=0.208), dissection at the proximal stent edge (7.9% vs. 6.3%, p=0.518), acute SM >200 µm (48.9% vs. 52.7%, p=0.337), or in-stent plaque/thrombus prolapse (29.8% vs. 27.2%, p=0.109) were not associated with an increased incidence of adverse events (Table 3).
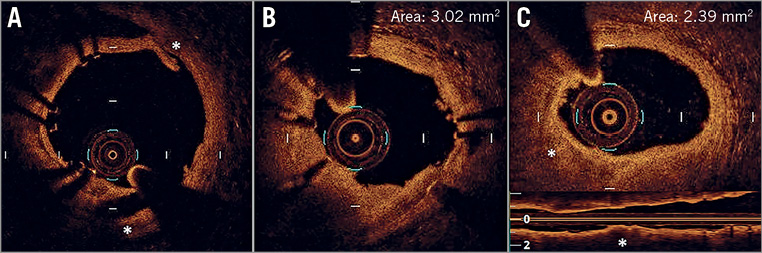
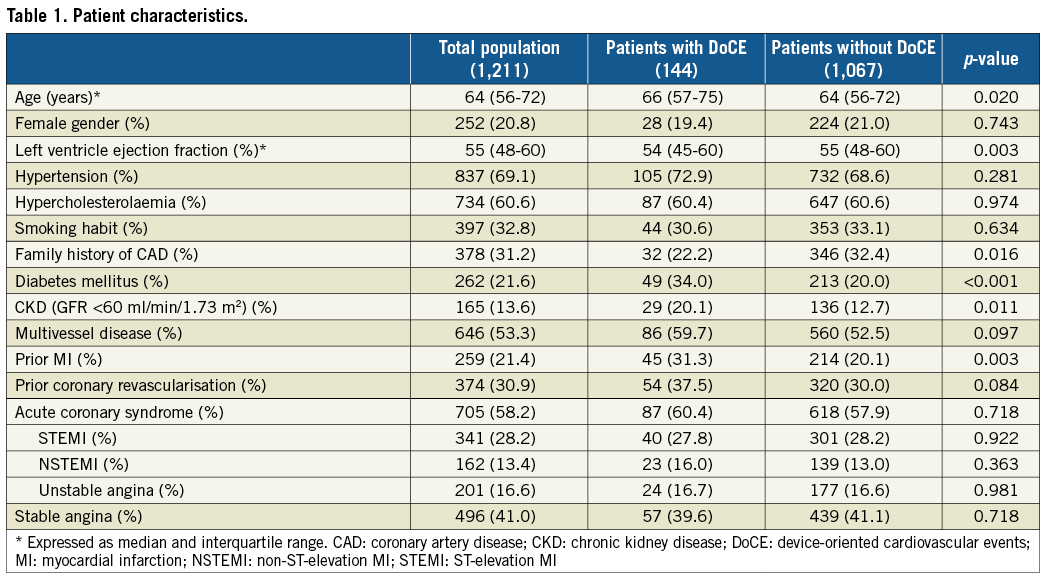
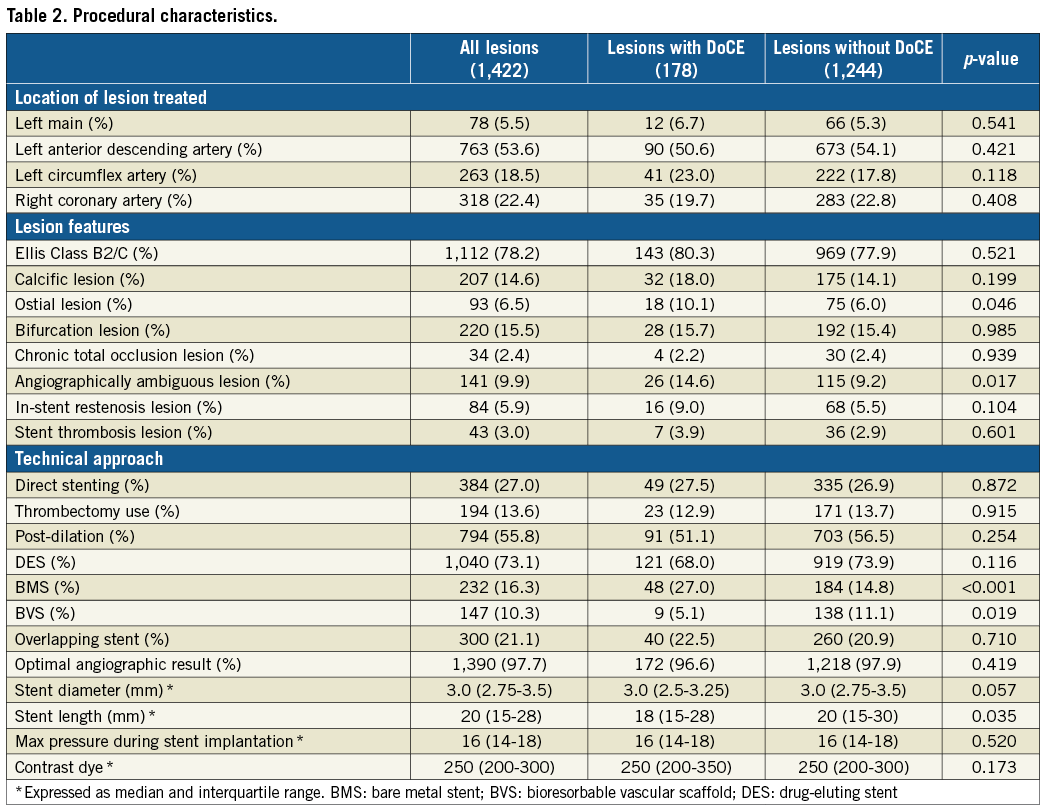
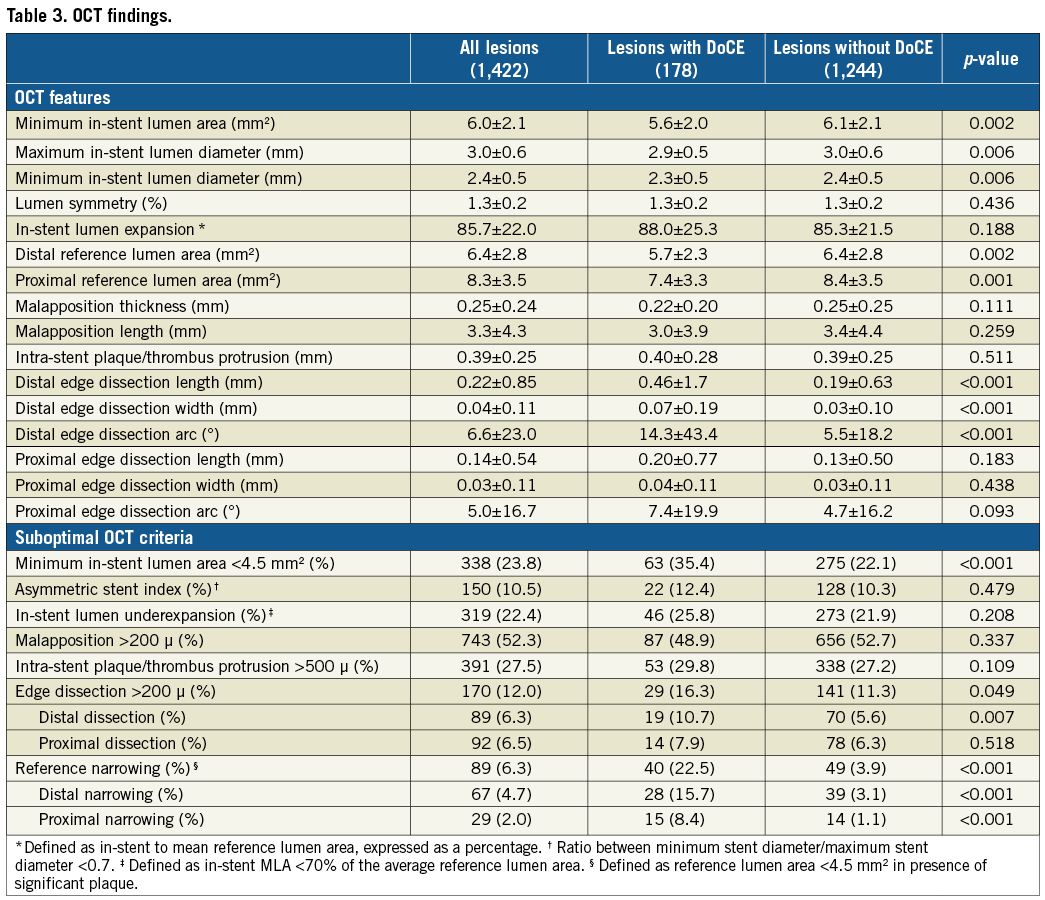
Figure 1. OCT criteria applied to address suboptimal OCT stent deployment. A) Edge dissection (*), defined as a linear rim of tissue with a width ≥200 μm and a clear separation from the vessel wall or underlying plaque, that was adjacent to a stent edge. B) Intra-stent minimum lumen area <4.5 mm2. C) Reference lumen narrowing defined as lumen area <4.5 mm2 in the presence of significant plaque (*) adjacent to stent edges.
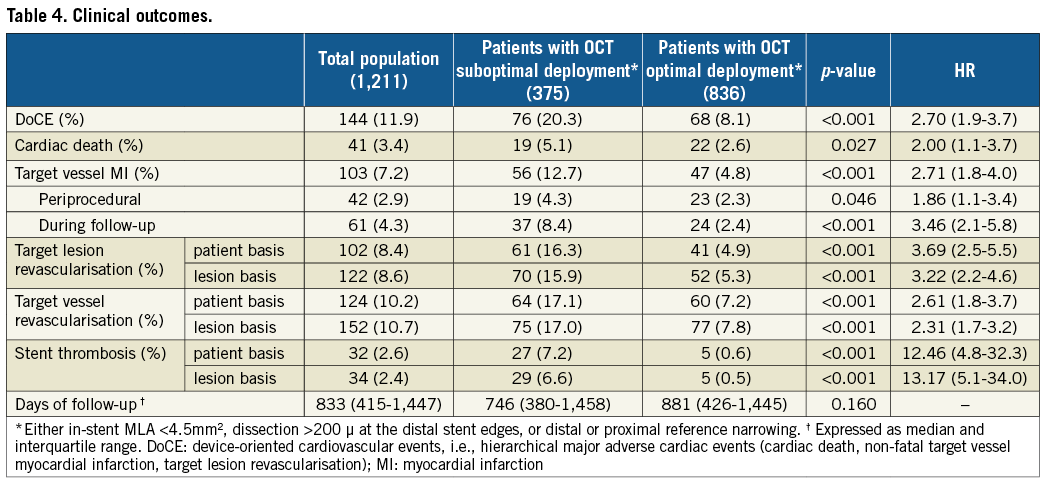
Cumulatively, the presence of at least one of the predictive OCT parameters (i.e., in-stent MLA <4.5 mm2, distal stent edge dissection >200 µm, and reference narrowing) was disclosed at the end of the procedure in 30.9% of treated lesions (Table 5) and was significantly more frequent in patients experiencing DoCE during follow-up (48.9% vs. 28.4%, p<0.001). The impact of a suboptimal OCT-defined stent implantation was confined to the early phases of follow-up (HR 2.52, 95% CI: 1.4-4.6, p=0.002), while the clinical outcome was comparable after the first year (HR 1.47, 95% CI: 0.9-2.5, p=0.165). A Kaplan-Meier curve of the incidence of DoCE is shown in Figure 2.
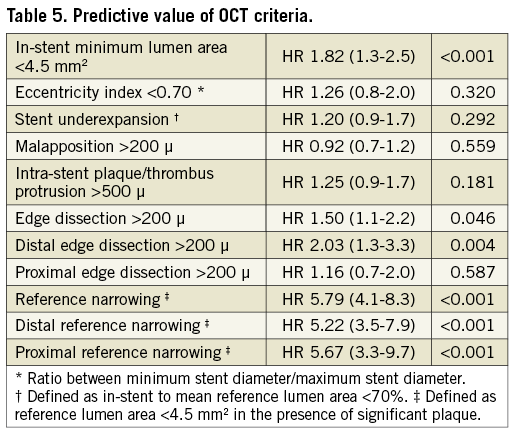
Figure 2. Clinical outcome. Time-to-event curves for device-oriented cardiovascular events (DoCE) according to optimal vs. suboptimal stent deployment assessed using OCT. DoCE: composite of cardiac death, target vessel myocardial infarction, and target lesion revascularisation (TLR)
In the multivariable Cox hazard analysis, suboptimal OCT stent deployment was confirmed as an independent predictor of long-term DoCE (HR 1.92, 95% CI: 1.3-2.9, p=0.001), together with diabetes mellitus (HR 1.89, 95% CI: 1.2-2.9, p=0.003), chronic kidney disease (HR 1.67, 95% CI: 1.1-2.7, p=0.030), and ostial lesion location (HR 2.57, 95% CI: 1.4-4.7, p=0.002).
Discussion
This study validates, in an adequately large real-world population, the clinical impact of an OCT-defined suboptimal stent implantation at long-term follow-up. In particular, our data confirm the DoCE reduction associated with OCT guidance over standard angiography evaluation during stent implantation, extending to a longer observation period (about three years) the earlier conclusions of the CLI-OPCI project7. Interestingly, the same OCT features that were found to be predictive at one-year follow-up remained significantly related to long-term DoCE, with reference narrowing being the metric with by far the highest clinical impact.
THE CLI-OPCI PROJECT AND EFFECTIVE METRICS OF SUBOPTIMAL STENTING
The CLI-OPCI project was designed with the goal of systematically collecting from high-volume OCT-experienced centres data on all consecutive PCIs performed with intraprocedural OCT assessment. The clinical usefulness of OCT-specific metrics was originally tested in the CLI-OPCI registry (335 patients) that showed a clinical benefit at one year in terms of cardiac death and myocardial infarction with OCT guidance6. Subsequently, the larger CLI-OPCI II study (832 patients) identified OCT metrics of suboptimal stent deployment (in-stent MLA <4.5 mm2, dissection >200 µm at the distal stent edge, and reference lumen area <4.5 mm2 in the presence of residual significant plaque at stent edges) as being associated with worse outcome in the first year of follow-up7.
The current CLI-OPCI LATE study enrolling a total of 1,211 patients with a median follow-up of almost three years aimed to evaluate the clinical benefit of an optimal OCT-defined stent implantation at long-term follow-up. A cumulative DoCE rate of 11.9% was recorded with an incidence of cardiac death (3.4%), target vessel-related MI (7.2%), and TLR (8.6%) consistent with other large all-comer PCI registries19. Despite the satisfactory angiographic result, the end-procedural OCT assessment revealed a significant rate of suboptimal stent deployment (30.9% of lesions) that was significantly higher in patients experiencing DoCE (48.9% vs. 28.4%, p<0.001). In fact, patients with at least one of the predictive OCT criteria showed an increased risk of cardiac death (HR 2.0), target vessel MI (HR 1.86 for periprocedural and HR 3.46 for post-discharge MI), and TLR (HR 3.22). After correction for other potential confounding factors and comorbidities, the presence of a final suboptimal stent deployment was confirmed as an independent outcome predictor (HR 1.92). Notably, all predictive OCT metrics identified in the CLI-OPCI II – in-stent MLA <4.5 mm2, dissection >200 µm at the distal stent edge, and reference vessel disease with lumen area <4.5 mm2 – remained associated with a worse outcome, albeit their predictive strength was maximal during the first year of follow-up (Table 5, Figure 2). The observed attenuation of the OCT clinical impact with a stabilisation of survival curve divergence after the first year probably reflects the emerging influence of the baseline clinical risk profile and worsening of atherosclerosis that became more evident at long-term follow-up.
NON-EFFECTIVE OCT METRICS OF SUBOPTIMAL STENTING
According to the one-year data of previous CLI-OPCI registries6-8, and also as seen in the present study, acute residual SM, variably observed in over 50% of stents, was not significantly related to the risk of long-term stent failure (HR 0.92). Albeit the lack of an OCT follow-up is a limitation in discussing the role of acute SM as a potential cause of stent thrombosis due to incomplete stent coverage20,21, the present OCT findings (consistent with the ADAPT-DES IVUS data22), seem to suggest a safe management of acute residual SM with single antiplatelet therapy after the first year23.
Similarly, % stent narrowing (i.e., relative stent underexpansion), considered the most important metric to address the adequacy of stent deployment in most randomised studies24,25, was not related to a worse clinical outcome in the current analysis (HR 1.20). This finding, probably due to lumen/calibre mismatch between diseased and healthy reference segments, should be considered when designing future randomised studies in order to assess the clinical utility of OCT guidance.
Limitations
The retrospective design represents the main limitation of the present study. Indeed, this registry included patients with different clinical conditions uniquely pooled by intraprocedural OCT use (not randomised). Nevertheless, after correction for the evident clinical and procedural differences, the presence of non-optimal OCT criteria for stent deployment was confirmed as an independent predictor of DoCE in the multivariable Cox hazard analysis.
Starting from the generally adopted IVUS/OCT definitions of suboptimal stent deployment, in the present study, and in the CLI-OPCI registries6-8, we proposed luminal cut-offs to delineate a practical approach to OCT guidance. However, this approach needs further clinical validation from randomised studies as the role and importance of the described OCT findings could vary according to different patient/device categories.
Conclusions
The presence of a suboptimal stent deployment, defined according to specific quantitative OCT criteria, was confirmed as an independent outcome predictor at long-term follow-up. These data add more evidence to the clinical utility of an OCT-guided strategy during PCI.
| Impact on daily practice In the context of a large multicentre real-world registry (the Centro per la Lotta Contro l’Infarto – Optimisation of Percutaneous Coronary Intervention project), this study demonstrated a correlation between optical coherence tomography (OCT)-defined suboptimal stent deployment and worse long-term clinical outcome. In particular, the presence of distal stent edge dissection >200 µm, in-stent minimum lumen area <4.5 mm2, and significant reference vessel plaque with a lumen area <4.5 mm² at the stent edges, are independent predictors of device failure. Larger randomised prospective studies are needed to confirm the clinical impact of OCT guidance during percutaneous coronary intervention and to investigate the management and the possibility of improvement of OCT-defined suboptimal stent deployment. |
Appendix. CLI-OPCI project study investigators
Executive committee: Francesco Prati, MD (principal investigator and chair), Ospedale San Giovanni - Addolorata, Rome, Italy; Gary S. Mintz, MD, Cardiovascular Research Foundation, New York, NY, USA; Fernando Alfonso, MD, Hospital Universitario de la Princesa, IIS-IP, Madrid, Spain.
Steering committee: Corrado Tamburino, MD, Azienda Ospedaliera Universitaria Ferrarotto, Catania, Italy; Francesco Burzotta, MD, PhD, Università Cattolica Del Sacro Cuore, Rome, Italy; Enrico Romagnoli, MD, PhD, Ospedale San Giovanni - Addolorata, Rome, Italy; Laura Gatto, MD, Ospedale San Giovanni - Addolorata, Rome, Italy; Ugo Limbruno, MD, Ospedale della Misericordia, Grosseto, Italy; Eloisa Arbustini, MD, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Clinical sites and investigators: Valeria Marco, RT, Centro per la Lotta Contro L’Infarto, Rome, Italy; Chiara Russo, RT, Centro per la Lotta Contro L’Infarto, Rome, Italy; Alessio la Manna, MD, Azienda Ospedaliera Universitaria Ferrarotto, Catania, Italy; Giovanni Ruscica, MD, Azienda Ospedaliera Universitaria Ferrarotto, Catania, Italy; Italo Porto, MD, PhD, Università Cattolica Del Sacro Cuore, Rome, Italy; Giampaolo Niccoli, MD, PhD, Università Cattolica Del Sacro Cuore, Rome, Italy; Carlo Trani, MD, Università Cattolica Del Sacro Cuore, Rome, Italy; Filippo Crea, MD, PhD, Università Cattolica Del Sacro Cuore, Rome, Italy; Nevio Taglieri, MD, Policlinico Sant’Orsola-Malpighi, Bologna, Italy; Francesco Saia, MD, Policlinico Sant’Orsola-Malpighi, Bologna, Italy; Silvio Fedele, MD, Azienda Ospedaliera Sandro Pertini, Rome, Italy; Francesco Versaci, MD, Ospedale Santa Maria Goretti, Latina, Italy; Francesco Amico, MD, Presidio Ospedaliero Sant’Elia, Caltanissetta, Italy; Salvatore Azzarelli, MD, Presidio Ospedaliero Sant’Elia, Caltanissetta, Italy; Vito Ramazzotti, MD, Ospedale San Giovanni - Addolorata, Rome, Italy; Alessandro Manzoli, MD, Ospedale San Giovanni - Addolorata, Rome, Italy; Fabrizio Imola, MD, Ospedale San Giovanni - Addolorata, Rome, Italy; Alessandro Pappalardo, MD, Ospedale San Giovanni - Addolorata, Rome, Italy; Mario Albertucci, MD, Ospedale San Giovanni - Addolorata, Rome, Italy; Franco Fabbiocchi, MD, Centro Cardiologico Monzino IRCCS, Milan, Italy; Giuseppe Z. Calligaris, MD, Centro Cardiologico Monzino IRCCS, Milan, Italy; Luca Di Vito, MD, PhD, Azienda Ospedaliera Mazzoni, Ascoli Piceno, Italy; Alessandro Di Giorgio, MD, Policlinico G. Martino, Messina, Italy; Antonio Bracco, MD, Policlinico G. Martino, Messina, Italy; Alberto Boi, MD, Ospedale Brotzu, Cagliari, Italy; Angelica Rossi, MD, Ospedale Brotzu, Cagliari, Italy; Marco Contarini, MD, Presidio Ospedaliero Umberto I, Syracuse, Italy; Giorgio Sacchetta, MD, Presidio Ospedaliero Umberto I, Syracuse, Italy; Antonio Trivisonno, MD, Ospedale A. Cardarelli, Campobasso, Italy; Irene Pescetelli, MD, Ospedale A. Cardarelli, Campobasso, Italy; Andrea Picchi, MD, Ospedale della Misericordia, Grosseto, Italy; Paolo Calabria, MD, Ospedale della Misericordia, Grosseto, Italy; Massimo Fineschi, MD, Azienda Ospedaliera Universitaria Senese, Siena, Italy; Elisabetta Iardino, MD, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Funding
The CLI-OPCI project has been entirely sponsored by the Centro per la Lotta Contro l’Infarto – Fondazione Onlus, Rome, Italy, with no extramural funding.
Conflict of interest statement
F. Prati has served as a consultant for St. Jude Medical. G. Mintz has served as a consultant for or has received honoraria from Boston Scientific, Volcano, ACIST, and Infraredx. F. Burzotta has received speaker’s fees from Medtronic, Abiomed, and St. Jude. The other authors have no conflicts of interest to declare.

