Abstract
Aims: The objective of this study is to evaluate the feasibility and safety of imaging human coronary arteries in vivo by optical frequency domain imaging (OFDI) in comparison to intravascular ultrasound (IVUS). OFDI has been recently developed to overcome the limitations of conventional time-domain optical coherence tomography (OCT), namely the need for proximal balloon occlusion. The Terumo-OFDI system is capable of acquiring images with high-speed automated pullback (up to 40 mm/sec) and requires only a short injection (3 - 4 sec) of small amount of x-ray contrast (9-16 ml).
Methods and results: Nineteen patients who underwent stent implantation were enrolled. IVUS/OFDI were performed before and after stenting. The incidences of any adverse event and angiographic adverse findings were recorded. Lumen area (LA) was measured by IVUS and OFDI at 1 mm intervals in the stented segments (n=19) as well as in the proximal, distal, and to-be-stented segments (n=40). In addition, lumen area in the stented segment was also measured by edge (E-) and video-densitometric (VD-) quantitative coronary angiography (QCA). The OFDI images were obtained without any adverse event related to imaging procedures. Post stenting (n=19), minimal LA (MLA) measured by OFDI (5.84±1.89 mm2) was larger than that of E-QCA (4.16±1.46 mm2, p<0.001) and VD-QCA (4.92±1.55 mm2, p<0.05). It was smaller than IVUS-MLA (6.26±2.01 mm2, N.S.) but the correlation between the two measurements was highly significant (R2=0.82, p<0.001).
Conclusions: The OFDI imaging is feasible both before and after stenting and has a promising safety profile. The OFDI provided clear high resolution images and robust lumen measurements.
Abbreviations
IVUS: intravascular ultrasound
OFDI: optical frequency domain imaging
QCA: quantitative coronary angiography
MLA: minimal lumen area
Introduction
Intravascular optical coherence tomography (OCT) has been introduced as an alternative high-resolution imaging method1-3 to intravascular ultrasound (IVUS). The latter is considered as the gold standard in measurement of vessel dimensions4. The spatial resolution of OCT is approximately 10 to 20 µm (about 10 times higher than IVUS).
Recently, the application of frequency-domain ranging techniques to OCT has further increased the imaging speed. Feasibility of this second generation OCT system, termed Fourier-domain OCT (FD-OCT) or optical frequency domain imaging (OFDI), has already reported5,6. These reports have shown potential advantages of FD-OCT/OFDI over conventional time-domain OCT systems, i.e., resulting in less angina and transient electrocardiogram (ECG) changes. However, there is no comparison of quantitative measurements between FD-OCT/OFDI and IVUS. Optical frequency domain imaging developed by Terumo is capable of acquiring images at a rate of 160 frames/s (pullback speed 20 mm/s) compared to 20 frames/s for a standard OCT system (pullback speed 1-3 mm/s). This fast pullback should help to widen the clinical applicability of OCT acquisition.
The objectives of this study are to assess 1) the ability to obtain images of the coronary artery pre and post stenting utilising fast pullback without need for vessel occlusion (features of OFDI technology) and 2) a qualitative and quantitative comparison of OFDI versus IVUS.
Methods
Study design and endpoints
This first in man prospective, single centre, feasibility study of the Terumo intravascular OFDI system (Terumo Corp., Tokyo, Japan) was performed to establish safety and assess the performance when imaging the coronary artery before and after stent deployment. The local ethics committee approved the protocol. All patients gave written informed consent before procedure. In total nineteen patients were enrolled, and were randomised in a 1: 1 fashion to either OFDI followed by IVUS (n=10) or IVUS followed by OFDI (n=9) in order to eliminate potential bias in safety outcomes due to sequence of two imaging techniques.
Patients were eligible if they had no more than two-vessels requiring stent implantation. If a patient had two epicardial coronary stenoses, the second lesion was eligible for the study once the first most severe lesion had been treated without any procedural event. The target lesion for imaging had to have a reference diameter no larger than 5.0 mm with a minimum luminal diameter (MLD) greater than 1.5 mm as measured by quantitative coronary angiography (QCA) before imaging and treatment. If the MLD was smaller than 1.5 mm, balloon predilatation was mandatory before imaging. Patients who had unprotected left main coronary artery disease and ostial right coronary artery disease were excluded. ST elevation myocardial infarction within 72 hours was also an exclusion criterion.
Primary endpoints were: 1) ability to obtain OFDI images of coronary artery pre- and post-stenting by OFDI with fast pullback, short flush and without proximal vessel occlusion and; 2) safety of OFDI procedure as assessed by occurrence of serious adverse events during the use of the device (i.e., coronary dissection, coronary spasm, development of thrombus in the artery subjected to imaging, clinical and/or electrocardiogram [ECG] evidence of ischaemia requiring treatment during imaging, coronary occlusion, occurrence of MACE [cardiac death, myocardial infarction, target vessel revascularisation] up to 30 days follow-up).
Secondary endpoints were length of imaged segment, comparative measurement of luminal dimension with IVUS and QCA.
Study device
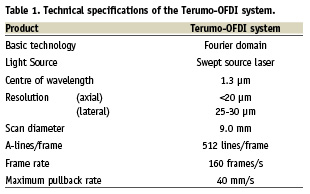
The Terumo OFDI system uses a scanning laser as light source of which centre wavelength is around 1.3 µm with sweeping range over 100 nm. In order to create the OFDI images, the interference between the tissue sample and a fixed reference mirror is processed to determine the echo-time delay and the amplitude of light reflected from the tissue microstructure at different depths. The technical specifications of this system is shown in Table 1. The imaging console permits real-time data processing and the two-dimensional representation of the backscattered light is displayed in a cross-sectional image. The catheter is connected at its proximal end to the motor drive unit. The OFDI catheter has a short monorail design and contains the fiberoptic core that rotates inside a translucent sheath. The OFDI imaging element rotates at 9,600 rpm allowing imaging at 160 frames/s. The pullback speed can be changed from 5 to maximally 40 mm/s. In the current study, the images were acquired at an automated pullback speed of 20 mm/s.
Imaging procedures
The procedure was performed via a 6 Fr guiding catheter. After intracoronary (IC) administration of nitroglycerin (≥0.1 mg), coronary angiography was performed. When the MLD was less than 1.5 mm on baseline quantitative angiogram, predilatation was performed. Both OFDI and IVUS imaging were performed in a single target lesion before and after stenting with postdilatation if judged necessary by the operator. The sequence of imaging for the devices IVUS/OFDI was randomised in the cathlab by the envelope method. Each imaging procedure/pullback was preceded by the intracoronary administration of 0.2 mg nitroglycerin. Coronary “safety” angiograms were performed in-between pullbacks with both IVUS and OFDI to monitor for adverse events.
OFDI imaging
Under fluoroscopic guidance, the OFDI catheter was advanced into the vessel, placing the optical lens ≥5 mm distal to the lesion or the stent. Imaging calibration was performed using the outer border of the catheter circumference (0.8 mm). OFDI pullbacks were performed during continuous injection of a maximum of 16 ml of 100% contrast medium (Iodixanol 320, Visipaque™, GE Health Care, Cork, Ireland) through the guiding catheter using an injection pump (Mark-V ProVis, Medrad Inc. Indianola, PA, USA). The contrast was injected at a flow rate of 3 to 4ml/sec for a maximum of 4 sec. The same procedure was repeated before and after stenting. During the entire procedure, continuous electrocardiographic (6 leads) and haemodynamic monitoring were carried out. The images were saved in the console and then converted into AVI files. The images were exported for off-line analysis.
IVUS imaging
IVUS imaging was performed using a commercially available 3.2 Fr, 40 MHz ultrasound catheter (Atlantis SR, Boston Scientific, Natick, MA, USA). The IVUS catheter was advanced into the vessel, with the imaging device placed at least 5 mm distal to the lesion or the stent. Automated pullbacks were acquired at 0.5 mm/s over the entire length of the target lesion/stent. All IVUS images were exported into DICOM standard format for off-line analysis.
Risk assessment
Procedural outcomes of both imaging procedures were compared. All cardiac complications occurring during the procedure and 24 hours postprocedure were reported. Major complications were defined as myocardial infarction (more than twice the upper limit of normal for creatine kinase (CK) with a presence of elevated CK-MB), emergency revascularisation, or death. Angiographic complication such as acute vessel occlusion, dissection, thrombus formation, embolism, or vasospasm along the entire target artery, and significant arrhythmias requiring immediate treatment were documented in a detailed case record form (CRF).
Analysis
An independent core laboratory reviewed all coronary angiograms, OFDI and IVUS images (Cardialysis BV, Rottedam, The Netherlands). In all imaging modalities, three different regions were identified: stented segment and the 5 mm-proximal and -distal to the stent. The QCA measurements included percentage diameter stenosis, lesion length, minimal lumen diameter, maximal lumen diameter, reference vessel diameter and minimal lumen areas (MLA) (CAASII, Pie-Medical, Maastricht, The Netherlands). Two different MLA measures based on videodensitometry and edge-detection techniques were analysed7,8).
For the IVUS and OFDI analyses, one frame per millimetre was selected and analysed independently by IVUS and OFDI analysts double blinded to the other technique. For IVUS quantitative analysis, a commercially available computer-based contour detection programme was used (CURAD BV, Wijk bij Duurstede, The Netherlands), whereas the OFDI analysis was performed using a customised and dedicated offline software (QCU-CMS, MEDIS medical imaging systems BV, Leiden, The Netherlands)9.
For pre-stenting images, the “to-be-stented segment” was defined as the region that was subsequently stented. This region was selected looking at the post-stenting recording and superimposing the boundaries of the stent on the vessel prior to the treatment by using side branch or vessel calcification as landmark, which is a standard operational procedure of the corelab10. In addition, regions immediately adjacent 5 mm proximal and distal to this segment were analysed. Lumen area (LA) and stent area (SA) were measured in the stented segments and LA was measured in the proximal, distal and to-be-stented segments. Mean LA and SA were calculated in each segment. The presence of thrombus, dissection, prolapsed tissue and incomplete stent apposition (ISA) were assessed in the segment according to previously described criteria11-13 and the incidence of these findings was expressed as the percentage of the segments with such findings divided by the total number of segments. If thrombus was present, thrombus area was subtracted from LA.
To assess inter-observer variability of LA measurements in OFDI images, analyses of three stented segments were repeated by two independent observers. To determine intra-observer variability, the images were analysed again by the same two observers at least four weeks after the initial measurement, and these two measurements were compared.
Statistics
No formal sample size calculation was performed for the feasibility study. The co-primary endpoint “ability to obtain OFDI images of coronary artery” was scored in the CRF by the investigator. For the co-primary safety endpoint of the OFDI imaging procedure, the rates of complications observed by angiography or ECG following the procedure were calculated as the number of patients with at least one event of the specified type divided by the total number of patients in the intention-to-treat group.
For the comparison of OFDI with IVUS, the measurements obtained from the OFDI and IVUS images, minimal and mean cross sectional lumen/stent area, are analysed using repeated measures ANOVA. The analysis included the segments which had both the OFDI images and corresponding IVUS images. The agreement of the matched OFDI and IVUS regions of interest was analysed by Bland-Altman plots14. All tests were two-sided and p-values <0.05 were considered statistically significant.
Results
Patients and image acquisition
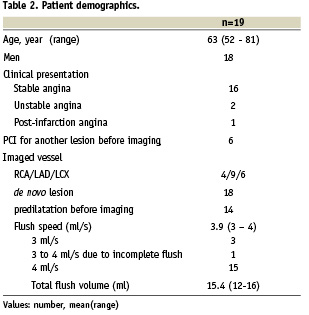
Patient baseline demographics are summarised in Table 2. IVUS/OFDI before stenting was not attempted in three patients, who were excluded from pre-imaging evaluable group due to lower than 1.5 mm MLD, but were included in the current intention-to-treat analysis. In the remaining patients OFDI catheter was positioned successfully distal to the target lesion before and after stenting. In summary, 16 sets of OFDI/IVUS pullbacks were obtained before stent deployment while 20 sets were acquired after stenting. During OFDI acquisition, angiographic contrast medium was injected at a mean flow rate of 3.9 ml/s (range 3-4 ml/s), with an average volume per pullback of 15.4 ml (range 12-16 ml).
Adverse events
There were no adverse events related to the imaging procedure. A total of six adverse events were definitely related to PCI procedure and detected by coronary angiogram. Three patients had angiographic transient side branch occlusion after stenting. Two of them did not present with permanent sequelae. In the third patient, transient ST segment elevation was seen in the catheterisation laboratory and a rise in Troponin T was reported postprocedure. Two patients had coronary dissection after predilatation. In one patient, in-stent thrombus was observed in angiography after stent deployment. None of the patients experienced adverse events within 30 days after procedure except for one patient who underwent a non-target lesion revascularisation for in-stent restenosis.
Matching OFDI and IVUS
Two OFDI pullbacks (one before and one after stent) were not analysable due to severe artefact related to an inadequate connection to the motor drive unit. One OFDI pullback (before stenting) was not analysable due to incomplete blood clearance, and one OFDI pullback (before stenting) could not be matched due to incomplete imaging in the distal segment. Eventually, 13/16 (81%) OFDI pullbacks before stenting and 19/20 (95%) OFDI pullbacks after stenting could be matched to IVUS pullbacks. In 32 pullbacks, average segment length imaged was 61±17 mm for IVUS and 52±17 mm for OFDI. Despite a small difference in pullback lengths, both techniques imaged 32 identical matched regions of interest demarcated by anatomical landmarks such as side branches seen on coronary angiogram or pre-procedural IVUS or OFDI.
Quantitative measurements were performed in a total of 59 analysable IVUS/OFDI matched segments acquired in 32 pullbacks (stented segments: n=19, proximal, distal, to-be-stented segments: n=40). In stented segments, mean LA and MLA by OFDI were larger than those measured by QCA and smaller than those measured by IVUS (Table 3). Mean stent area measured with OFDI was also smaller than that measured with IVUS, while incomplete stent apposition (ISA) area determined by OFDI was larger than that by IVUS.

In stented, proximal, distal, to-be-stented segments and their pooled analysis, mean LA and MLA measured by OFDI were highly correlated with those measured by IVUS (Figures 1 and 2). In stented segment, both mean LA and MLA by OFDI tended to be smaller than those measured by IVUS (p=0.104 and p=0.102, respectively). In proximal, distal, to-be-stented segment, both mean LA and MLA by OFDI were significantly smaller than those by IVUS (both p<0.001). The limits of agreement for all these parameters are reported in Figures 1 and 2.
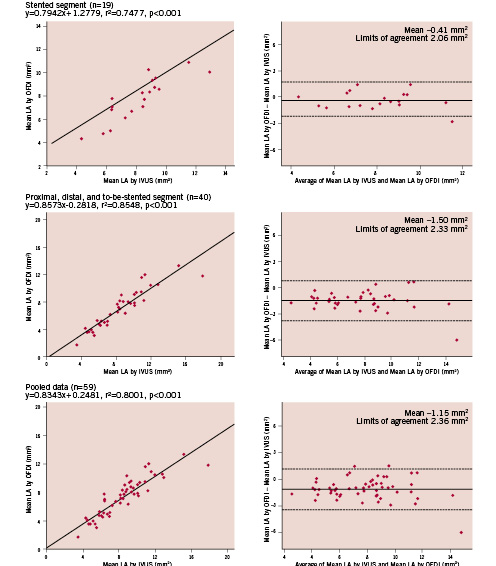
Figure 1. Regression analysis and Bland-Altman analysis for mean lumen area (LA).

Figure 2. Regression analysis and Bland-Altman analysis for minimal lumen area (MLA).
Figure 3 shows MLA sites corresponding on IVUS and OFDI. OFDI visualised irregular lumen contour. Corresponding cross sectional images obtained in other cases illustrate the difference in qualitative assessments observed between OFDI and IVUS (Figure 4). Tissue prolapse and ISA can be recognised both in IVUS and OFDI, but these are more clearly visible with OFDI than with IVUS, and intraluminal defects attached to stent struts are more readily detectable on OFDI than on IVUS. Table 4 shows ability of two systems to detect thrombus, tissue prolapse, dissection and incomplete stent struts apposition.
The significant difference between IVUS and OFDI was found.
The intra-observer variance and coefficient of variation for lumen area on frame level as measured by quantitative OFDI was 0.0016 mm2 and 0.0052, respectively. The inter-observer variance and coefficient of variation for lumen area on frame level was 0.0003 mm2 and 0.0024, respectively.
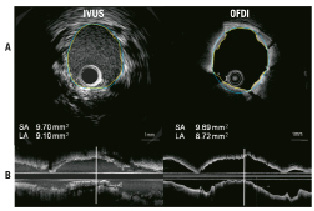
Figure 3. IVUS/OFDI corresponding cross sectional and longitudinal images. A: Minimal lumen area site, OFDI can clearly visualise irregular lumen contour due to tissue prolapse. Lumen area (LA) by OFDI is smaller than LA by IVUS. SA: stent area. Vertical solid lines on longitudinal images (B) indicate corresponding sites of cross sectional images. Yellow and blue lines on A indicate LA and blue line indicates SA, respectively.
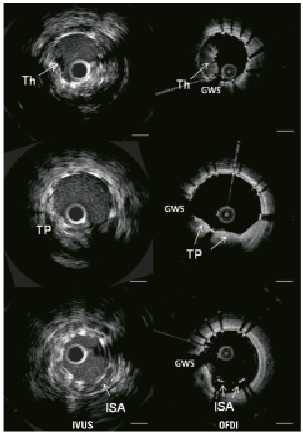
Figure 4. IVUS/OFDI corresponding cross sectional images. Th: thrombus; TP: tissue prolapse; ISA: incomplete stent apposition; GWS: guidewire shadowing; Bar=1 mm

Discussion
The main findings of this first-in-man OFDI study are the following: 1) the OFDI images were obtained without any adverse events related to the imaging procedure, 2) mean and minimal LA were smaller with OFDI than with IVUS, although the correlations between two modalities were high.
Safety and feasibility
This study demonstrates the safety of OFDI imaging: there were no adverse events related to the OFDI imaging procedure in patients with the minimal lumen diameter pre-stenting was larger than 1.5 mm, as per protocol inclusion criteria. The absence of severe adverse events is in line with the previous findings reported with conventional TD-OCT methodology. In the multicentre study by Barlis et al15 including 468 patients who underwent conventional TD-OCT imaging with non-occlusive method using a maximal pullback speed of 3 mm/s, major complications such as ventricular fibrillation and air embolism were reported to occur in 0.9% and 0.5% of the patients, respectively, while self-limited phenomena such as chest pain and transient ST elevation occurred more frequently (20.8% and 1.9%, respectively). Takarada et al reported that the commercially available FD-OCT system (LightLab Imaging C7XR; LightLab Imaging Inc., Westford, MA, USA) provided a safer acquisition with a better image quality than conventional TD-OCT system5. More specifically, ST elevation and chest oppression was more frequently observed with conventional OCT than FD-OCT (ST elevation: 0 vs. 10%, P <0.05, chest oppression: 5 vs. 70%, P <0.001).
Short acquisition time of OFDI image with reduced amount of injected contrast medium obviously contributes to the absence of ischaemic events observed in this trial as previous reports5,6. With OFDI, the image acquisition time is less than 4 sec (80 mm of pullback length). In addition, the small amount of the angiographic contrast medium –similar to the amount used during conventional coronary angiogram– explains the absence of ST/T change or chest pain during the imaging procedure. The mean contrast volume used in this trial was 15.4 ml (range 12-16 ml) per pullback, while the contrast volume in conventional TD-OCT investigation with non-occlusive technique is approximately 40 ml per pullback15. The contrast volume needed to remove blood might be reduced when the pullback speed will achieve up to 40 mm/s. Further study is necessary to evaluate the feasibility of optimal pullback speed of this OFDI system.
Quantitative assessment
The comparison in lumen area measurement between QCA, OFDI and IVUS indicates that there exists a systematic difference in area assessment ranging from the smallest to the largest measurement: QCA (edge detection: ED), QCA (video densitometry: VID), OFDI and IVUS. The lumen dimensions with OFDI in this study are systemically smaller than IVUS dimensions in both stented and non-stented segments, however, the magnitude of differences is greater in non-stented segment than in the stented segment. In the present study the inter- and intra- observer variances were very low for OFDI quantitative analysis suggesting high reproducibility of the quantitative OFDI analysis. Previous reports also described excellent agreement for measurements of lumen area16.
Measurement of smaller dimension with OCT than with IVUS, when analysing the same anatomic structure, has been repeatedly described in the literature. In a recent report by Capodanno et al, lumen areas in the stented segment at six month follow-up measured by OCT were compared to that by IVUS frame by frame using a sampling rate of 0.5 mm for both techniques17. Lumen area assessed with OCT images which were obtained using M2 system with a non-occlusive technique was significantly smaller than IVUS by 0.22 mm2 (relative reference –5.4%). In a study by Gonzalo et al18, the lumen dimensions of ex vivo human coronary arteries were compared between IVUS, OCT and histomorphometry. When ex vivo OCT and IVUS were compared, lumen measurements obtained with OCT were smaller than IVUS by 11%. Noteworthy, when OCT was performed in a plexiglass phantom manufactured with a precision of 10 µm, the OCT measurement correlated extremely well with the real lumen dimension (relative standard deviation 1.8%, r=1.000, intercept 0.01, slope 1.02)19.
The differences between OFDI and IVUS observed in this trial can be multifactorial:
1) They can be related to the specific backscattering of either sound or light in human arteries. In other words, the recognition of luminal boundary might be inherently different due to physical characteristics of the wave lengths used by these technologies. Both in in vitro and in vivo setting, IVUS measurement is influenced by blood flow velocity, blood temperature, eccentric catheter placement20. OFDI can also be influenced by eccentric or concentric catheter position, but might be less influenced by blood flow velocity, since the blood is removed during OFDI imaging acquisition by injection of contrast media with a constant flow rate19. The potential vasoconstrictive effect of contrast medium injected at room temperature vs. blood or contrast at 37ºC could also differentially affect the dimension of stented vs. non-stented segment and potentially explain the larger and more significant difference in luminal dimension observed between the two imaging modalities in the non-stented segments. In addition, an increased shear stress from the rapid injection induce vasoconstriction (instead of the normal vasodilatation) in these abnormal diseased segment. This does not apply to the stented segment hence the difference.
2) They can be due to difference in resolution between the two modalities (OFDI has a resolution of <20 µm while IVUS 100 µm), OFDI may more clearly visualise intra-luminal/intra-stent structures such as tissue prolapse and thrombus11, which would consequently be subtracted from OFDI luminal area measurement, possibly resulting in smaller luminal dimensions when assessed with OFDI than with IVUS. In the present study, however, stent area and lumen area were similar in each modality. Thus, this impact might be small.
3) The third possible explanation is the differential effect of cardiac cycle. With IVUS pullback speed of 0.5 mm/sec, image acquisition takes usually 30-50 sec and images are acquired over many cardiac cycles (±72 heartbeats/min). With IVUS measurement, the difference in luminal dimension between systole and diastole can bias the measurement by 12%21. On the other hand, because of the fast pullback speed of 20 mm/s, image acquisition with OFDI takes only 3-4 sec with no more than a few cardiac cycles. Since OFDI cannot select specifically systolic or diastolic images and also include systolic images, assessment of the lumen dimensions is a sequential mixture of two or three systolic and diastolic images. This may explain the larger difference between IVUS and OFDI in the non-stented segments (Figure 1). Furthermore, average longitudinal transducer movement of IVUS catheter during cardiac cycle as measured by IVUS is 1.50±0.80 mm (range 0.5 to 5.5 mm)22. Without gating, IVUS image sequence may be a non-sequential mixture of cross-sectional images23. These mechanical considerations have implication for the outcome of measurements analysed.
The Bland-Altman approach is a widely used method when comparing measurement agreement of two quantitative techniques imaging the same object14. It requests the same exact number of data points (frames) for comparison, a count that could be hampered by the different acquisition rate of the two imaging techniques compared. The OFDI acquires images at 160 frames/sec with a pullback speed of 20 mm/s thus providing 8 frames per mm (0.125 mm/frame). IVUS, on the other hand, acquires images at 30 frame/sec with a pullback speed of 0.5 mm/s, providing 60 frames per mm (0.016 mm/frame). For all the reasons mentioned above, it is not surprising that the number of IVUS/OFDI frames recorded in the same stent differs, rendering frame-to-frame analysis problematic. Consequently, we have opted for a segment analysis of the stent (stented segment: n=19, non-stented segment: n=40, pooled: n=59).
Limitations
This study was based on a relatively small and selected population. Although this study was not designed to detect potential clinical advantages of higher resolution of OFDI, it is interesting to note that there were significant differences in detecting ability of thrombus, dissection, tissue prolapse, and incomplete stent struts apposition. Larger study population will be necessary to prove such advantage.
Conclusion
Using this high-speed OFDI system, image acquisition in the stented and non-stented segment was as feasible as IVUS imaging and was not associated with any complications. Furthermore, OFDI provided robust and reproducible lumen measurements, which correlated with IVUS measurements, although quantitative measurements with OFDI were systematically smaller than with IVUS.
Acknowledgements
We thank Dragica Paunovic, MD and Vladimir Borovicanin, MD, employees of Terumo Corporation and Terumo Europe N.V. for supervising the conduct of the trial and for their helpful technical and administrative support and advice. We thank Monique Schuijer, PhD, Pierre Gobbens, MSc, Koen Commissaris, MSc from Cardialysis, who have coordinated the trial and statistically analysed the data. We thank Scot Garg, MD and Nico Bruining, PhD who gracefully reviewed the manuscript and provided the authors with thoughtful criticism and comments.

