Introduction
The large imaging depth of intravascular ultrasound (IVUS) combined with the high spatial resolution of optical coherence tomography (OCT)/optical frequency domain imaging (OFDI) offers refined insight into coronary atherosclerosis1,2. The integration of IVUS and OCT/OFDI into a single hybrid imaging catheter has previously been demonstrated in vivo in animals3 and in patients4. Here, we present an improved IVUS-OFDI system that removes critical barriers to the clinical adoption of dual-modality imaging. Our catheter connects to the console through a fast interchange interface for simple and reliable operation. A novel algorithm fuses the IVUS and OFDI signals into a single combined image for intravascular holistic structural visualisation (IV-HSV). We report on imaging experiments with a human cadaver heart and swine in vivo, demonstrating the unique benefits of this system.
Methods
Our imaging console operates concurrent 1,300 nm OFDI and 40 MHz IVUS imaging at 24.8 fps with a pullback speed of up to 20 mm/s. As displayed in the Central illustration, the clinical grade imaging catheters have a 2.6 Fr/3.2 Fr, 132 cm-long monorail design, compatible with standard 0.014” guidewires. The 1.3 mm-long rigid tip of the imaging probes comprises a side-viewing optical ball lens with a diameter of 200 µm and a 0.5 mm-wide ultrasonic transducer, placed 300 µm distally from the ball lens. The acoustic and optical beams are co-planar with respect to the catheter axis and feature an angle of 6˚ in the longitudinal direction, intersecting 2.8 mm from the sheath surface. A dual-channel rotary joint provides parallel optical and electrical connections and enables a mechanical locking mechanism to secure the dual-channel connection with a simple click-on, allowing fast interchange of the disposable catheters.
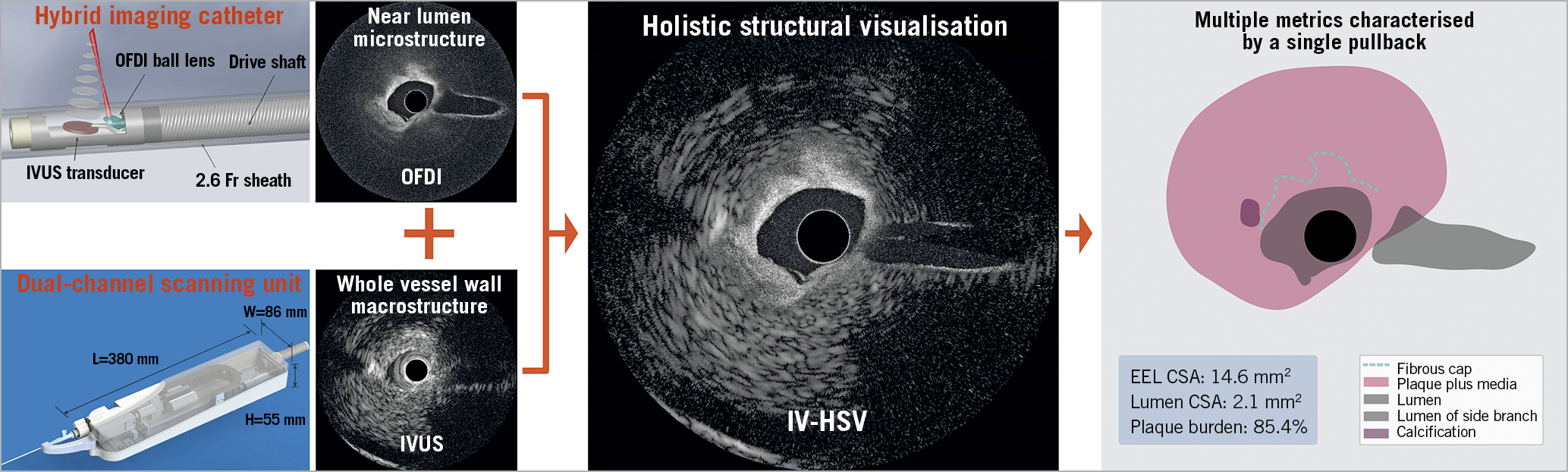
Central illustration. Intravascular holistic structural visualisation. Hardware and software work together to provide one complete map of vessel wall structures that can improve the diagnosis of coronary plaques during cardiac catheterisation procedures. (The pullback speed was 10 mm/s). CSA: cross-sectional area; EEL: external elastic lamina; IV-HSV: intravascular holistic structural visualisation; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging
To aid the interpretation of IVUS and OFDI, the co-registered images were accurately scaled to common spatial coordinates and computationally fused into a single structural map –IV-HSV– based on image content. Starting from the lumen, the algorithm seamlessly transitions from the OFDI signal to the IVUS signal at an optimal depth, automatically computed for each radial profile using an OFDI intensity threshold. This results in near-lumen structures being visualised with the high definition of OFDI, ideally complemented with the IVUS image of deeper lying features, overall preserving the most clinically relevant information.
Through the National Disease Research Interchange, a cadaver human heart was collected within 24 hours after death and stored at -80 degrees Celsius. Tissues were thawed before imaging experiments. Multiple coronary artery segments, including perivascular tissue, were resected from the heart. A custom fixture was used to mount the artery segments and ensure accurate co-registration with histopathology5. During pullback imaging, saline was manually flushed through the segments. After imaging, frozen sections of the arterial samples were processed for haematoxylin-eosin (H&E) and trichrome staining.
To test the system in a clinical setting, we conducted intravascular imaging in vivo in a healthy Yorkshire pig. A 7 Fr guide catheter was placed through the carotid artery to gain access to the coronary arteries. Both the left anterior descending artery (LAD) and left circumflex artery (LCX) were catheterised using a 0.014” guidewire. A total of fourteen 60 mm-long pullbacks were acquired at various pullback speeds (5-20 mm/s). Contrast injection was performed manually to displace circulating blood in the imaged coronary arteries.
Results
First, we performed dual-modality imaging of a human cadaver heart, to visualise various features of coronary atherosclerosis. Exemplary for fibroatheroma, the IV-HSV image in Figure 1 (panel A1) renders the lumen and the shallow fibrous cap as captured by OFDI, complemented by the outer border of the thick cap and the entire vessel wall cross-section as disclosed by IVUS. Notably, the vessel injury at 6-8 o’clock is visualised in high resolution with OFDI in IV-HSV, as it would be difficult to identify in IVUS. Furthermore, the IV-HSV image enabled evaluation of the external elastic lamina (EEL) cross-sectional area (CSA) owing to the IVUS signal. Although IVUS can also provide an estimation of luminal CSA, plaque burden estimation in IV-HSV is simplified by the clear lumen definition of OFDI. A fibro-calcified plaque is included in Figure 1 (panels B1-B6).
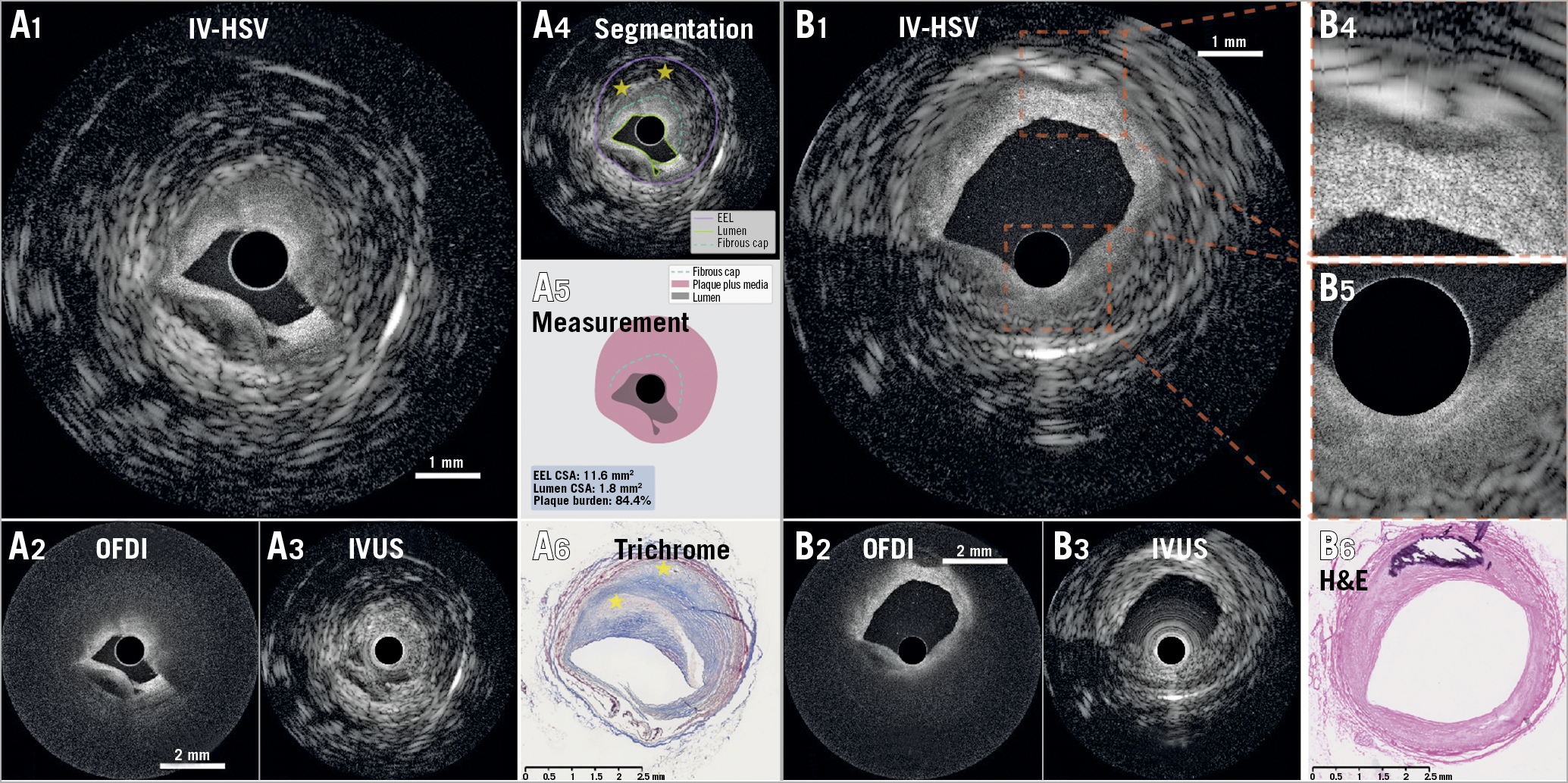
Figure 1. Representative holistic structural visualisation (IV-HSV) of a human cadaver coronary artery. Representative images of a fibroatheroma (A1 to A6) and a fibro-calcified plaque (B1 to B6). IV-HSV images (A1 & B1), combining the OFDI image (A2 & B2) and IVUS image (A3 & B3). A4) Segmentation of various plaque features in the IV-HSV image (A1), and isolated visualisation of the segmented structures, enabling computation of plaque burden (A5). A6) Matching trichrome-stained histopathology. A dissection of the coronary artery appears from 6 to 8 o’clock. The IV-HSV image (A1) visualises relevant details of the dissected vessel wall by optimally merging the IVUS and OFDI appearances. The yellow stars in panel A4 indicate necrotic cores, confirmed by histopathology (yellow stars in A6). Panels B4 & B5 show the magnified views of the square areas of interest indicated in panel B1. B6) Matching histopathology, stained with haematoxylin and eosin. Scale bars in panels A2 & B2 apply to both panels A2 & B2, and panels A3 & B3. The pullback speed was 10 mm/s. CSA: cross-sectional area; EEL: external elastic lamina; IV-HSV: intravascular holistic structural visualisation; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging
Next, we tested the use and performance of the dual-modality system in the catheterisation laboratory by imaging in a healthy swine (Figure 2). Owing to the intrinsic co-registration, OFDI and IVUS images show matching side branch locations. Luminal CSA measured independently with OFDI and IVUS reveal excellent Pearson’s correlation (r=0.97, p<0.001, N=40) (Figure 2B). The Bland-Altman plot (Figure 2C) suggests that neither the measurement bias (–0.07 mm2) nor the limits of agreement (0.25 and –0.38 mm2) are clinically important. The averaged Hausdorff distance and mean distance between the lumen borders segmented in both modalities are 160 μm and 56 μm with standard deviations of 48 μm and 22 μm, respectively6. This analysis suggests intrinsic co-registration of the two modalities, owing to the accurate alignment of the optical and ultrasound beams.
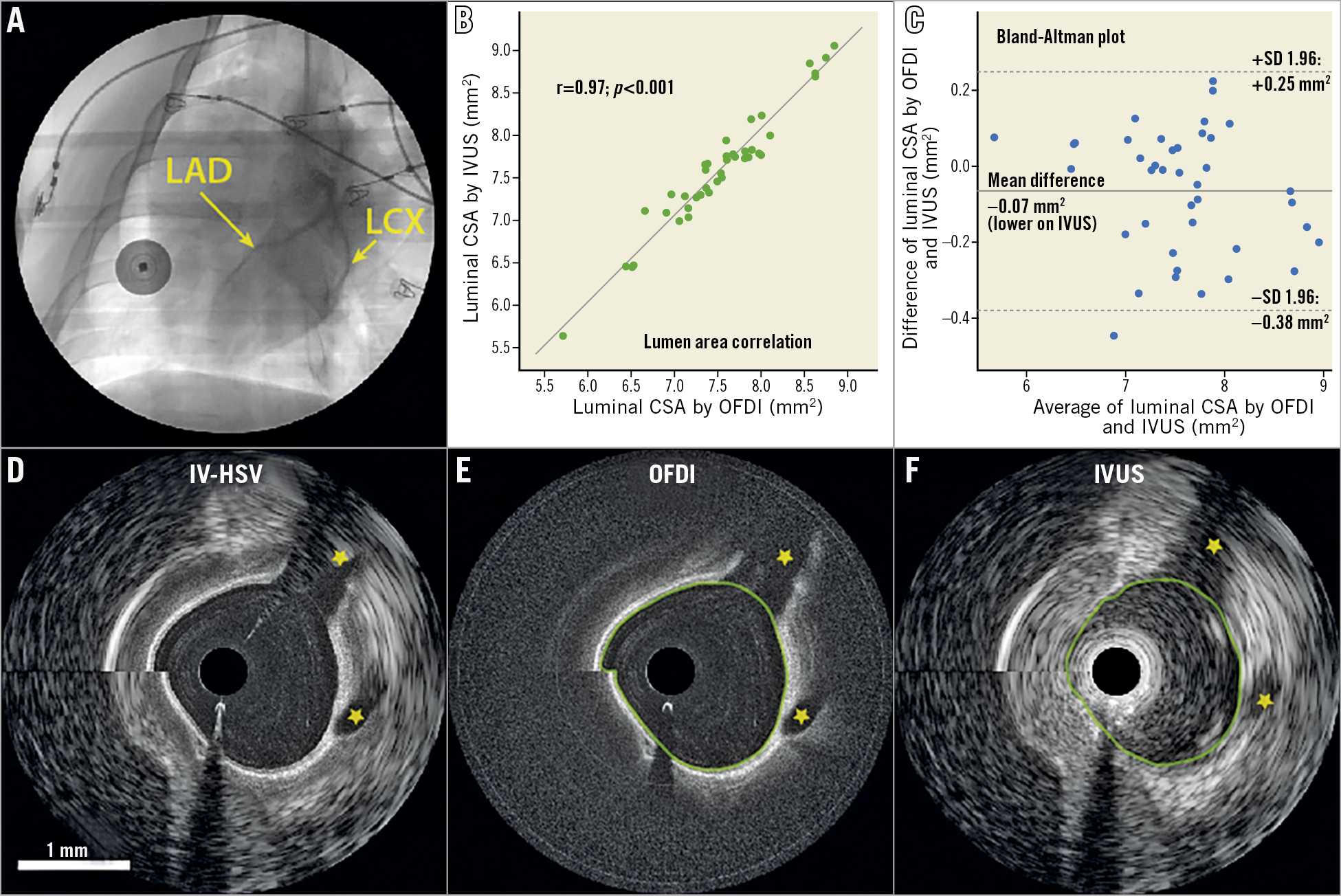
Figure 2. In vivo swine catheterisation and intravascular imaging. A) Coronary angiogram showing the left coronary arteries during pullback imaging. B) Pearson’s correlation analysis of 40 luminal CSAs measured independently with OFDI and IVUS across a 32 mm-long segment of the LAD with a large variation of lumen size and shape, acquired at 10 mm/s pullback speed. C) Bland-Altman plot of the 40 luminal CSAs measured independently with OFDI and IVUS. Panels D, E & F are intrinsically co-registered OFDI, IVUS, and cross-sectional IV-HSV images acquired in the LAD. The yellow stars indicate the locations of side branches. The “sew up” artefact at 9 o’clock is a result of the helical scan pattern of “rotation-pullback” imaging and heart motion during the intervention. Both panel B and panel C show excellent agreement between measurements with the two modalities, thanks to the accuracy of the intrinsic co-registration of the dual-modality imaging system. The green lines in panels E & F are the lumen segmentations. Panels D-F share the same scale bar as in shown in panel D. CSA: cross-sectional area; IV-HSV: intravascular holistic structural visualisation; IVUS: intravascular ultrasound; LAD: left anterior descending artery; LCX: left circumflex artery; OFDI: optical frequency domain imaging
Discussion
To date, dual-modality systems presented OCT and IVUS images either as separate panels, making spatial correlation across modalities difficult, or as colour-encoded overlay, which complicates thorough inspection as the layers shade each other. The large difference in resolution and speckle size between OCT and IVUS further aggravates this shading effect. In contrast, IV-HSV offers a single-colour, easy-to-comprehend view, enabling effortless interpretation of critical artery features that are relevant for guiding percutaneous coronary intervention (PCI).
As demonstrated by our results, this hybrid imaging system offers unique insights into vessel pathology. A first example is the simultaneous visualisation of both near-lumen microstructures and deep lesion morphology in a single map. In the IV-HSV of the Central illustration, a vessel injury at 7 o’clock was clearly imaged by OFDI and a deep calcification was disclosed by IVUS at 9 o’clock. A second example is the improved characterisation of calcification by the co-registered dual-modality imaging. While IVUS identifies deep calcification that OFDI alone is unable to detect (Central illustration), OFDI can assess the thickness of shallow calcification Figure 1 (panel B2), in addition to its length and arc angle. Calcium scoring including thickness parameters has been shown to predict stent expansion reliably7. Another possible benefit of IV-HSV is a potentially more accurate estimation of plaque burden than with either IVUS or OCT/OFDI alone, because it combines the robust mapping of the EEL owing to the deep penetration of IVUS with the accurate delineation of the lumen owing to the improved resolution of OCT/OFDI. This may refine plaque burden as a robust predictor of PCI outcomes8.
Limitations
Imaging of coronary atherosclerosis was performed in a single cadaver heart and a healthy swine was used for in vivo imaging. Clinical studies will be needed to demonstrate the diagnostic value of dual-modality imaging in a clinical setting. The fusion algorithm relies on an OFDI signal to define the transition from OFDI to IVUS, which could result in non-optimal partitioning of the two modalities due to artefacts in OFDI images. Finally, dual-modality imaging requires blood clearing through the injection of contrast media or saline for OFDI imaging.
Conclusions
This study demonstrates the technical capability of our dual-modality system and suggests its potential for aiding PCI. Acquired concurrently with a single pullback of a hybrid imaging catheter, intrinsically co-registered IVUS and OFDI images provide complementary diagnostic information. IV-HSV fuses them into a single-colour, easy-to-comprehend map, that provides interventionists with a holistic and accurate overview of the coronary arterial wall and its disease burden.
|
Impact on daily practice IVUS and OFDI are well-established intravascular imaging methods for guiding PCI, where IVUS visualises the entire vessel wall and OFDI images near-lumen microstructures at high resolution. Combining insights from independent imaging with IVUS and OFDI has been shown to improve the detection of high-risk plaques and the estimation of plaque structural stress, but is impractical in a routine clinical setting. Here, we present a catheter-based imaging system integrating IVUS and OFDI, which enables real-time IV-HSV of coronary arteries with a single catheter by automatically fusing IVUS and OFDI images in a single complete map of macro- and microstructures of the coronary arterial wall. |
Acknowledgements
K. Otsuka acknowledges partial support from the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad, the Uehara Memorial Foundation Postdoctoral Fellowship, and the Japan Society for the Promotion of Science Overseas Research Fellowship. The authors acknowledge Leon Ptaszek, MD, PhD, and Moussa Mansour, MD, at the Department of Cardiology, Massachusetts General Hospital for in vivo swine imaging, and Diane Tshikudi, MS, and Pallavi Doradla, PhD, at the Wellman Center for Photomedicine, Massachusetts General Hospital for their assistance in cadaver tissue preparation and histology.
Funding
This work was supported by the National Institutes of Health (P41EB015903, K99AG059946, and R01HL119065) and by the Terumo Corporation.
Conflict of interest statement
Massachusetts General Hospital has patent licensing arrangements with Terumo Corporation. B.E. Bouma and M.L. Villiger have the right to receive royalties as part of the licensing arrangements. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplementary data
To read the full content of this article, please download the PDF.

