Abstract
Background: In peripheral artery disease, two different types of calcification are frequently observed, i.e., medial and intimal calcification.
Aims: The aim of this study was to determine the ability of intravascular ultrasound (IVUS) imaging and optical frequency domain imaging (OFDI) to detect medial and intimal calcification in human peripheral arteries.
Methods: We performed ex vivo intravascular imaging of cadaveric human peripheral arteries with calcifications. IVUS and OFDI images were co-registered with histology. A total of 12 legs from nine patients were examined, and 438 cross-sectional images were co-registered with histology.
Results: OFDI could detect 183 of 231 intimal calcifications by histology, whereas IVUS could detect 194 (OFDI: sensitivity 79%, specificity 86%, area under the curve [AUC] 0.83; IVUS: sensitivity 84%, specificity 85%, AUC 0.85). Of 245 medial calcifications by histology, 160 and 164 were detected by OFDI and IVUS, respectively (OFDI: sensitivity 65%, specificity 85%, AUC 0.75; IVUS: sensitivity 67%, specificity 80%, AUC 0.74). Medial calcification with overlying intimal calcification (overlapped calcification) and an unclear border between intima and media were the main reasons for misdiagnosis. Without those 89 overlapped calcifications, sensitivity in both OFDI and IVUS was improved (OFDI: sensitivity 81%, specificity 85%, AUC 0.83; IVUS: sensitivity 88%, specificity 80%, AUC 0.84).
Conclusions: There are limitations in detecting medial calcification in overlapped intimal calcification and with an unclear border between intima and media by both IVUS and OFDI. It is important to distinguish medial calcification from intimal calcification before proceeding with endovascular therapy since different approaches will be required.
Introduction
Calcification is very common in atherosclerotic plaque in peripheral artery disease (PAD)1. The extent and degree of calcification is broadly used as a surrogate marker to predict clinical outcomes2. Typically, patients with PAD show more severe degrees of calcification relative to those with coronary artery disease1,3. There are two types of calcification, intimal and medial calcification1,3. The risk factors that contribute to these two types of calcification are different, although there is some overlap. Medial calcification has been characterised as a non-inflammatory degenerative disease development, independent of atherosclerosis since medial calcification is not affected by lipid deposition or inflammation which are typically associated with intimal calcification2. Medial calcification is first formed in elastic lamellae and extends to the adjoining media which is rich in smooth muscle cells. Diabetes mellitus (DM), and chronic kidney disease (CKD) are recognised as risk factors for medial calcification. Also, both intimal and medial calcification can occur in the same vessel wall due to a combination of the risk factors such as aging, DM and CKD2. Although medial calcification is not associated with luminal obstruction, the decrease in arterial vessel wall elasticity and compliance may ultimately lead to atherosclerosis, reduced perfusion and, eventually, PAD. Therefore, these two types of calcification require different approaches to treatment, and their differences need to be considered. Recently, three peripheral calcium scoring systems were proposed; however, all three classifications were categorised by the same grade, regardless of type of calcification4.
When selecting devices for endovascular therapy (EVT), or when seeking a better understanding of risk stratification, it is crucial to distinguish medial from intimal calcification using an intra-vessel imaging device such as intravascular ultrasound (IVUS) or optical frequency domain imaging (OFDI). To the best of our knowledge, there has been no study using intra-vessel imaging devices and histology as the gold standard to evaluate these two types of calcification in PAD. In this study, we assessed the detection of medial and intimal calcifications by 60 mHz-IVUS and OFDI with co-registered histological images.
Methods
Detailed methods are described in Supplementary Appendix 1.
STUDY ENROLMENT
Twelve legs with atherosclerotic disease from nine cadavers obtained from donors through Science Care (Phoenix, AZ, USA), with 21 arteries in total, were evaluated. The peripheral arteries above and below the knees (ATK and BTK) underwent 60 mHz-IVUS (AltaView™; Terumo Corporation, Tokyo, Japan) and OFDI (Lunawave®; also Terumo Corporation) with corresponding histologic sections and were enrolled in the study.
IMAGING PROCEDURE
Following gross examination of the legs, common femoral and popliteal arteries were cannulated and angiography was performed. Vessels were imaged by IVUS and OFDI. Each imaging run was performed at a pullback speed of 20 mm/s (frame rate 160 frames/s) for OFDI and 9 mm/s (frame rate 90 frames/s) for IVUS.
HISTOLOGICAL PROCESS FOR PERIPHERAL ARTERIES
Vessels were sequentially cut at 3 to 4 cm intervals after they were decalcified in ethylenediaminetetraacetic acid. The segments were embedded in paraffin following dehydration, as previously described, at 4 to 5 mm intervals and sectioned at 4 to 6 µm and stained by H&E and Movat’s pentachrome stains1. The segments without calcification on radiography were routinely embedded in paraffin for subsequent histochemical staining.
CO-REGISTRATION OF OFDI AND IVUS IMAGES WITH HISTOLOGY
All OFDI and IVUS images were co-registered with histologic sections by an experienced investigator (H. Jinnouchi). For careful co-registration between OFDI/IVUS images and histological sections, pullback speed, side branches, and distance from the nearest ostium were taken into consideration. Anatomical landmarks such as calcification and luminal configuration were considered to adjust images longitudinally and circumferentially.
CLASSIFICATION OF TYPES OF ATHEROSCLEROTIC LESION AND CALCIFICATION
All histology slides were reviewed by experienced readers (H. Jinnouchi and R. Virmani). Peripheral atherosclerotic plaques were classified using the modified American Heart Association classification proposed for coronary atherosclerotic lesions5. Histologically, different degrees of intimal and medial calcification and the size of the calcium were assessed1,2. Microcalcification was defined as calcium particles varying from >0.5 but <15 µm in diameter, punctate calcification as >15 µm but <1 mm, and fragment calcification as >1 mm. Sheet calcification was noted when >1 quadrant of the vessel demonstrated calcification. Nodular calcification was reported if the nodular calcium deposits in the atherosclerotic lesion had not disrupted the lesion’s luminal surface. Calcified nodule was defined as overlying thrombus with disruption of fibrous cap by underlying calcification. Overlapped calcification was defined as medial calcification with overlying intimal calcification with or without fusion. Considering the resolutions of OFDI and IVUS, any observed calcification was defined as punctate or a more severe type of calcification.
OFDI AND IVUS IMAGING ANALYSIS
Two OFDI and IVUS readers (Y. Sato and R. Bhoite) blinded to the histologic findings assessed the images independently. Figure 1, Figure 2 and the Central illustration show intimal and medial calcification by histology, OFDI, and IVUS. Calcification by OFDI was defined as a signal-poor or heterogeneous region with a sharply delineated border contrasted from the highly scattered or signal-rich intima. Media were defined as a low backscattering or signal-poor band bordered by a discrete high backscattering internal elastic lamina (IEL) that sat at the border of the intima and media. In OFDI images, intimal and medial calcifications were defined as calcification located in the intima or media. When the IEL and media are not clearly seen, information in adjacent sites is essential to identify the location of calcification in the intima or the media.
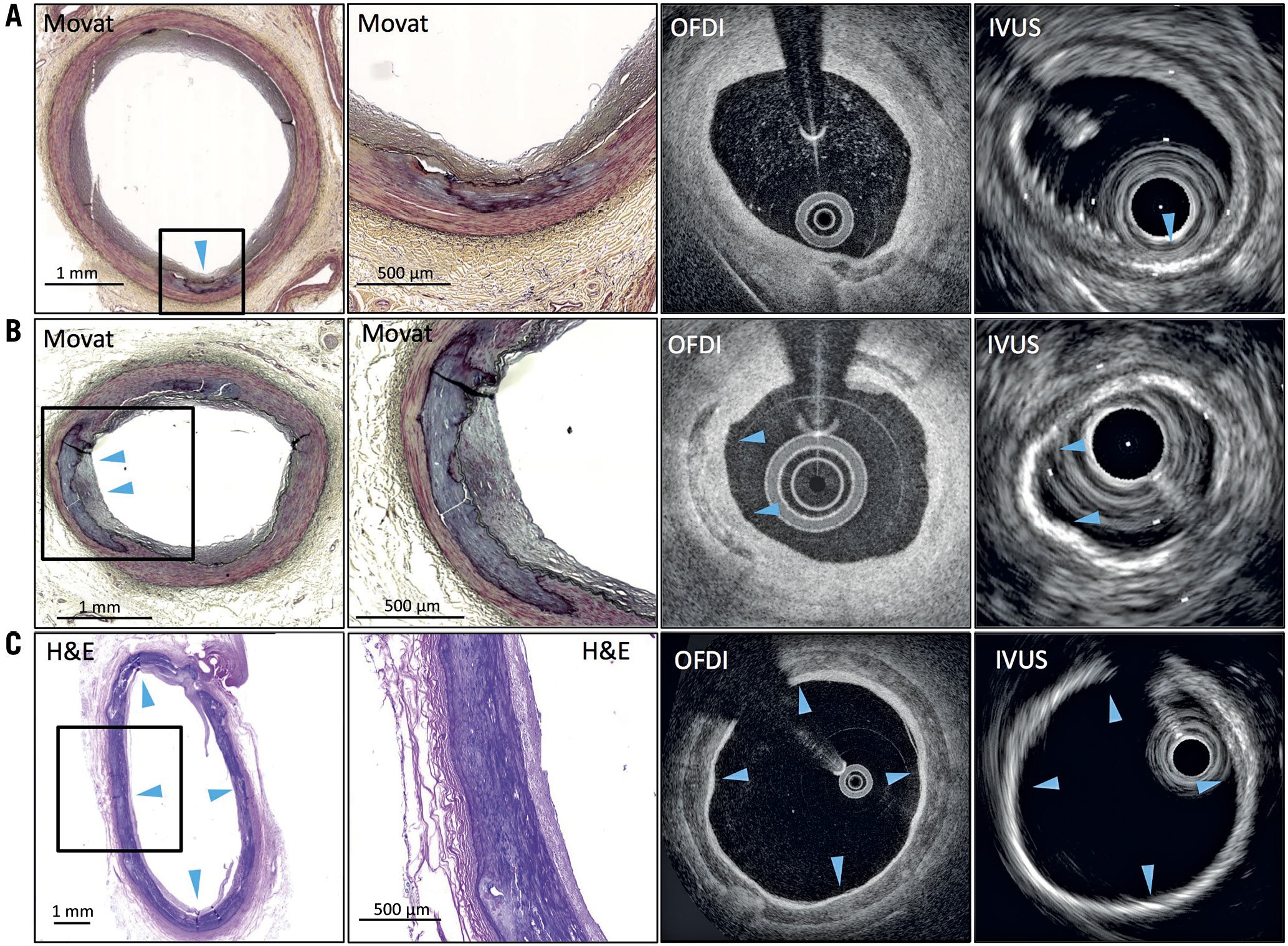
Figure 1. Representative images of medial calcification by IVUS and OFDI co-registered with histology. A) Medial punctate calcification. OFDI shows calcification with clear border (blue arrow). IVUS shows high echoic signal with a low acoustic shadow near the IEL. B) Histological section shows fragmented medial calcification. OFDI shows calcification in media, whereas IVUS shows a high echoic signal right under the IEL due to blooming artefact. C) Circumferential medial sheet calcification. OFDI shows circumferential calcification and IVUS shows high echoic signal with a strong acoustic shadow. Medial calcification (blue arrowheads). IEL: internal elastic lamina; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging
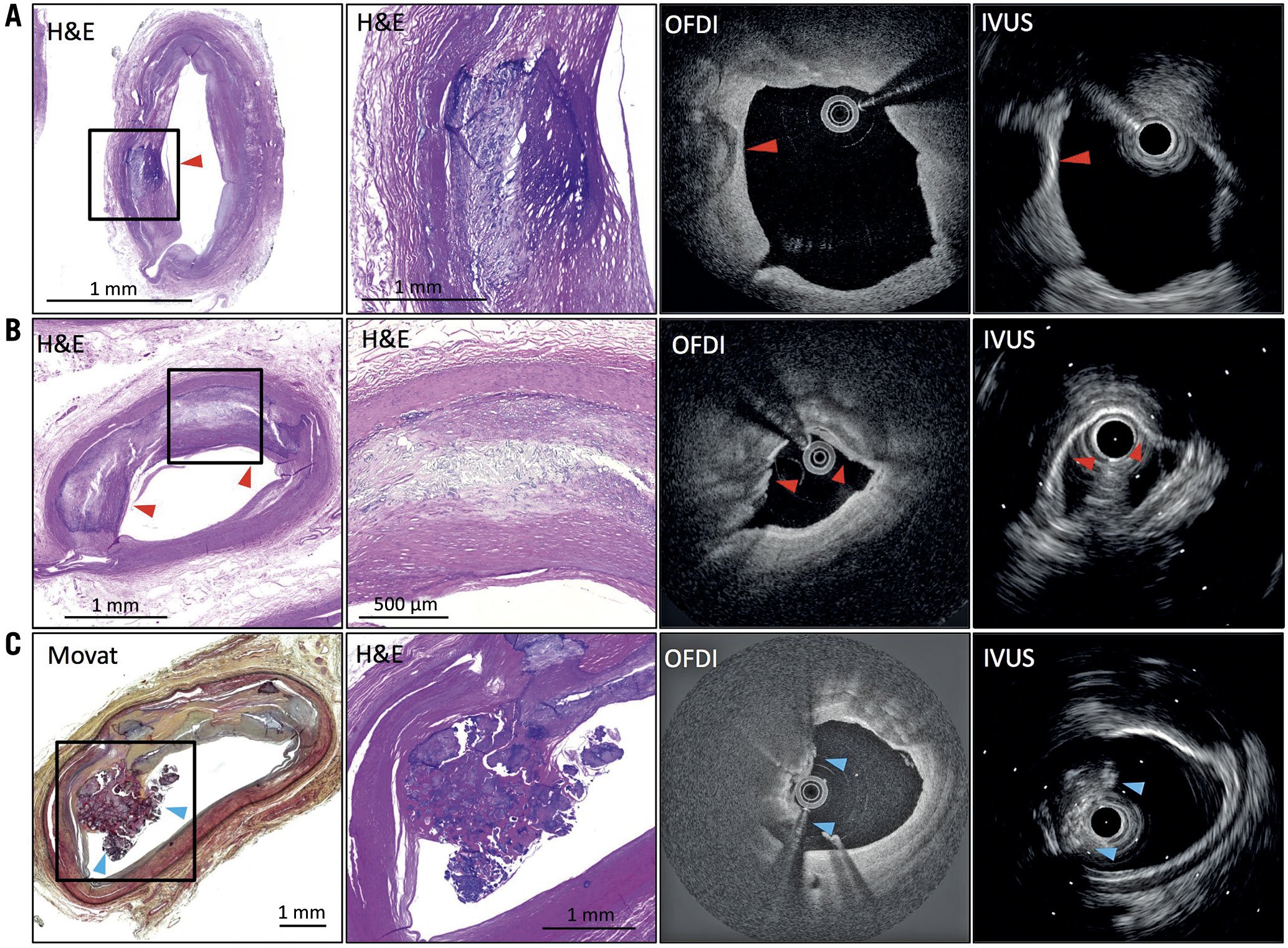
Figure 2. Representative images of intimal calcification by IVUS and OFDI co-registered with histology. A) Intimal fragmented calcification (red arrow). OFDI shows calcification with clear border only on the luminal side. IVUS shows high echoic signal with a strong acoustic shadow. B) A histological section shows superficial intimal calcification (red arrows). OFDI shows a clear border between intima and calcification; however, the abluminal edge of calcification is not visible due to its deep location. IVUS shows high echoic signal with a strong acoustic shadow derived from the presence of calcification. C) Intimal calcified nodule (blue arrows). There is no endothelium and collagen on the surface of the nodules. OFDI shows irregular protruded mass with attenuation signal in contact with large calcification. IVUS shows protruded and irregular mass with high acoustic shadow. IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging
![Central illustration. Differences in the longitudinal appearance of intimal versus medial calcification. A) Longitudinal images with X-ray on the left, micro CT highlighting calcification in the middle, and sagittal view of micro CT image in the right panel. Intimal calcification shows longitudinally thick calcification (yellow lines and brackets), whereas medial calcification shows a thin layer of calcification (red lines and brackets). B) Histologic images (H&E [left] and Movat’s pentachrome [right] stains), with corresponding OFDI and IVUS images showing intimal calcification (yellow arrows) corresponding to yellow lines in panel A. The panels on the extreme right show images corresponding to red lines from panel A, indicating medial calcification (red arrows), shown by histology, OFDI and IVUS. CT: computed tomography; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging](../../../storage/issues/EIJ196e/120_EIJ-D-20-01336_Jinnouchi_196e/medias/630014/00_Jinnouchi_196e.jpg)
Central illustration. Differences in the longitudinal appearance of intimal versus medial calcification. A) Longitudinal images with X-ray on the left, micro CT highlighting calcification in the middle, and sagittal view of micro CT image in the right panel. Intimal calcification shows longitudinally thick calcification (yellow lines and brackets), whereas medial calcification shows a thin layer of calcification (red lines and brackets). B) Histologic images (H&E [left] and Movat’s pentachrome [right] stains), with corresponding OFDI and IVUS images showing intimal calcification (yellow arrows) corresponding to yellow lines in panel A. The panels on the extreme right show images corresponding to red lines from panel A, indicating medial calcification (red arrows), shown by histology, OFDI and IVUS. CT: computed tomography; IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging
By IVUS, there are three layers. The intima is considered as the innermost layer, which is relatively echogenic compared with the media. The media is considered as the second layer, which is less echogenic than the intima. Calcification was defined as bright echoes that obstruct the penetration of ultrasound6. In IVUS images, intimal calcification was defined by the presence of these bright echoes in the intima. On the other hand, considering blooming artefact when medial calcification was involved with IEL, we defined medial calcification by IVUS as the presence of bright echoes over the media or within the media. When the media are not clearly seen, information in adjacent sites is essential to identify the location of calcification in the intima or the media.
Bone formation is composed of lacunae containing osteoblasts and bone marrow, typically in contact with sheet or fragmented calcification1. Bone formation by OFDI, which is another feature in PAD, was defined as the presence of a honeycomb sign (indicating bone marrow) in contact with calcifications (Figure 3). We also measured an area and arc of calcification by OFDI and IVUS.
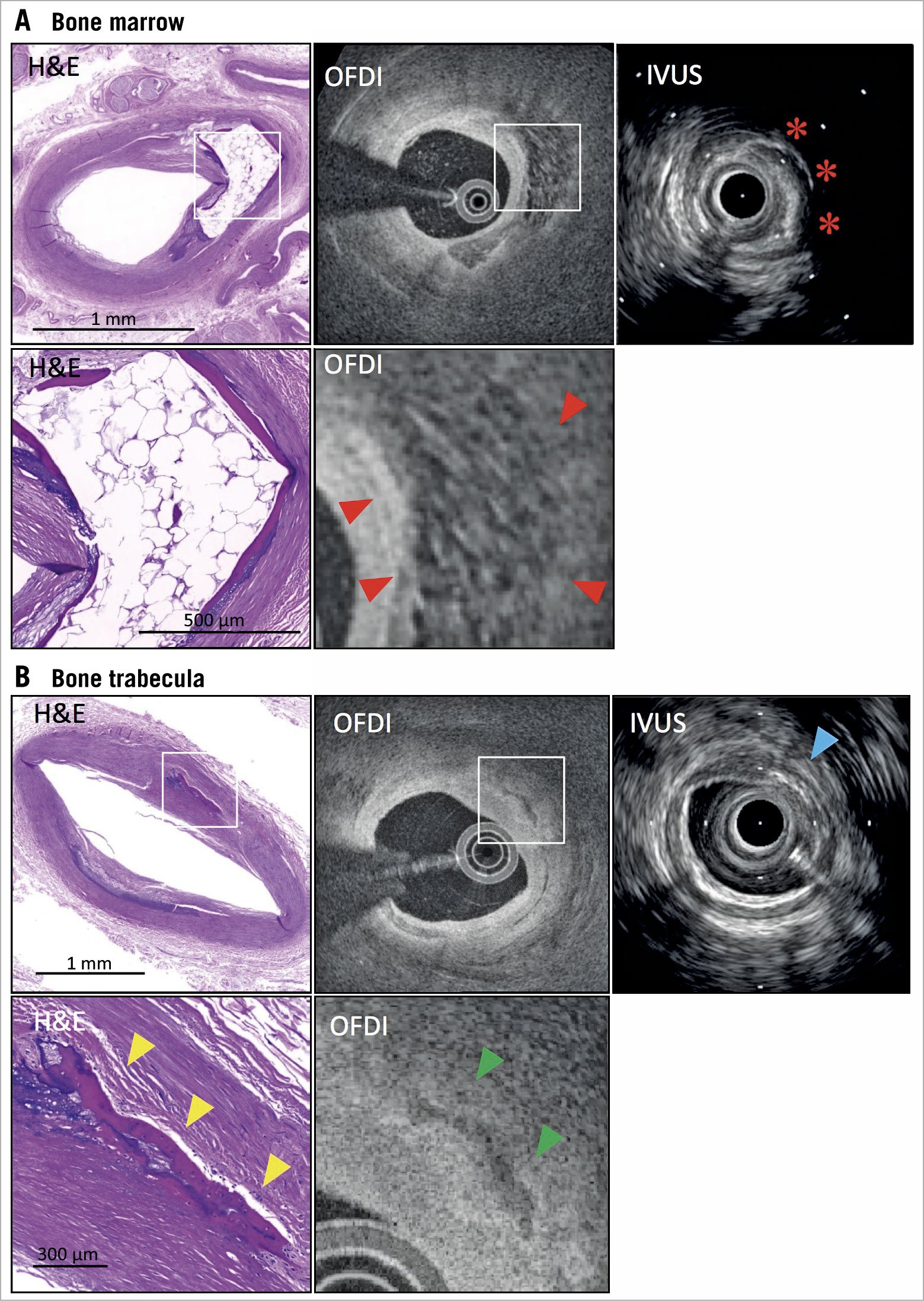
Figure 3. Findings of bone formation by IVUS and OFDI co-registered with histology. A) Histological image shows bone formation in the intima. High-power image shows thin trabecular bone at the edges of the bone marrow. OFDI shows honeycomb sign, indicating bone marrow best appreciated on high-power images (red arrowheads). IVUS shows high echoic signal with strong acoustic shadow (red asterisks). Lower panels show high-power images from white boxes in upper histological and OFDI panels. B) Histological image shows trabecular bone (yellow arrowheads) without bone marrow. OFDI shows irregular calcification (green arrowheads). IVUS shows high echoic sign (blue arrow) in intima-media border. Lower panels show high-power images from white boxes in upper histological and OFDI panels. IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging
STATISTICAL ANALYSIS
Continuous variables were expressed as means±standard deviation or median and interquartile range dependent on distribution of data after the normality of distribution was confirmed by the Shapiro-Wilk test. JMP software, version 13.0 (SAS Institute Inc., Cary, NC, USA), SPSS software, Version 19 (IBM Corp., Armonk, NY, USA) and MedCalc, version 19.7 (MedCalc Software Ltd, Ostend, Belgium) were used for statistical analyses.
Results
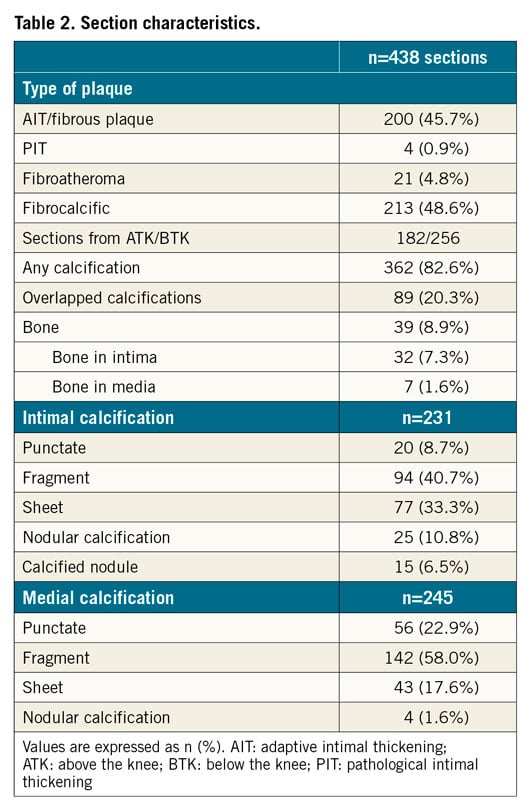
PATIENT AND SECTION CHARACTERISTICS
Table 1 lists case characteristics. Table 2 lists histological section characteristics. Processing artefacts (n=16) and 20 sections without either OFDI or IVUS images were discarded. Eventually, a total of 438 OFDI and IVUS images corresponding to histology were obtained. In histology, most sections (362 [83%]) showed calcification (intimal/medial calcification: 231 [53%]/245 [56%], respectively). Of 438 sections, 89 showed overlapped calcification. Types of calcification are shown in Table 2. Bone formation was found in a total of 39 (9%) sections (intima/media; 32 [7%]/7 [2%]). Medial calcification was significantly more frequently observed in BTK than ATK (Supplementary Figure 1, Supplementary Table 1). ATK showed a significantly higher prevalence of bone formation relative to BTK.
DIAGNOSTIC CAPABILITY FOR INTIMAL AND MEDIAL CALCIFICATION BY OFDI AND IVUS
Table 3, Supplementary Table 2 and Supplementary Figure 2 summarise sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy for the detection of intimal and medial calcification. Of 231 sections with intimal calcification, OFDI detected 183, but 48 were not detected, and 60 mHz-IVUS detected 194, but 37 were not detected (sensitivity/specificity/area under the curve [AUC], OFDI: 79%/86%/0.83, and IVUS: 84%/85%/0.85, respectively). Of the 245 sections with medial calcification, 160 were detected by OFDI, but 85 were not detected, and 164 were detected by IVUS, but 81 were not detected (sensitivity/specificity/AUC, OFDI: 65%/85%/0.75, and IVUS: 67%/80%/0.74, respectively) (Figure 4). Of the 85 and 81 sections that could not identify the type of calcification (i.e., medial or intimal) by OFDI and IVUS, respectively, overlapped calcification (in 55 and 62, respectively) was a main reason for undetected medial calcification, followed by an unclear border between intima and media (in 18 and 13, respectively), and small calcification (in 12 and 6, respectively) (Figure 5).
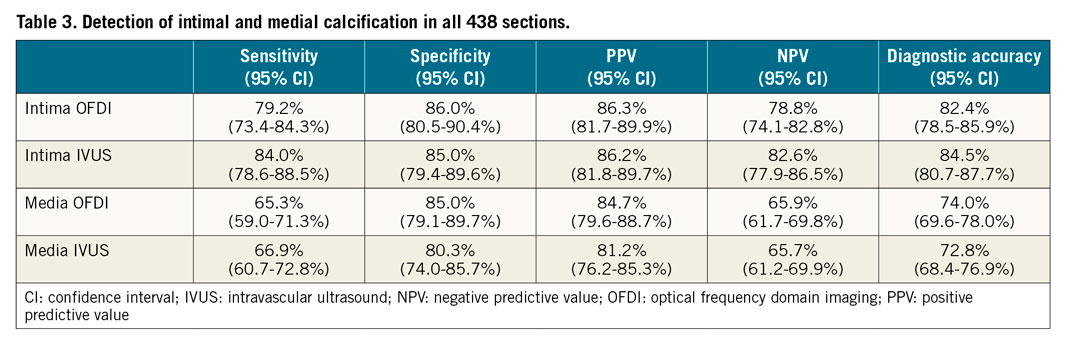
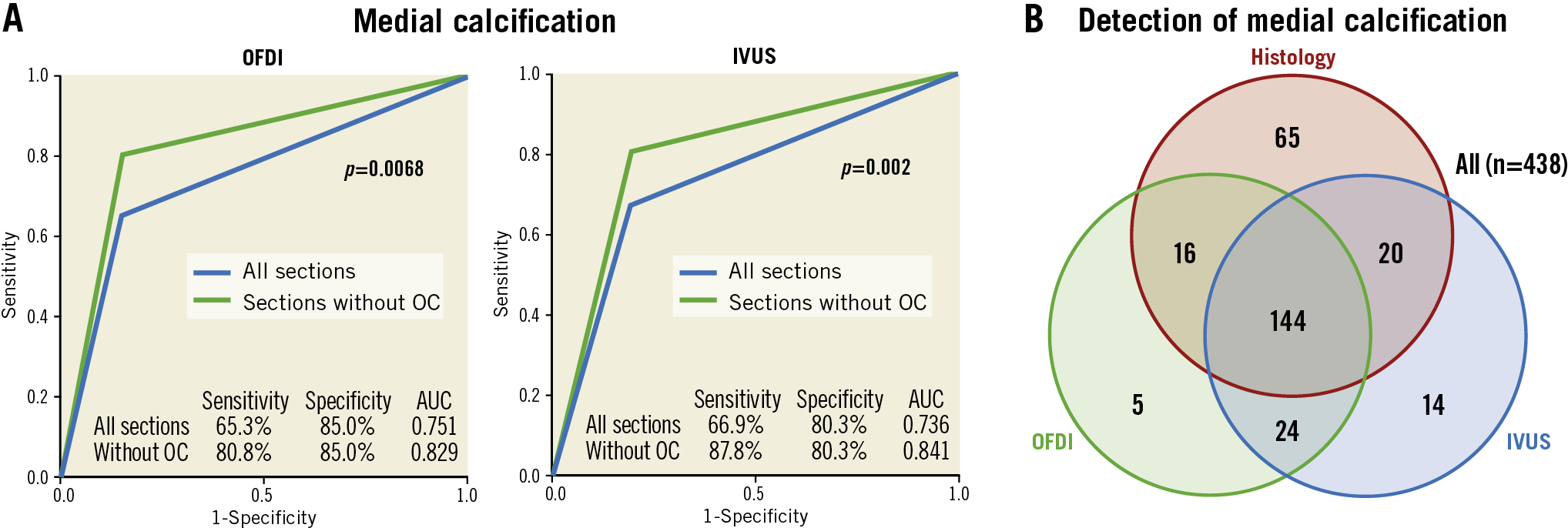
Figure 4. Detection of medial calcification by IVUS and OFDI. A) Receiver operating characteristic curve for detection of medial calcification in all sections and in sections without overlapped calcifications by OFDI and IVUS. B) Number of sections diagnosed by histology, OFDI and IVUS. AUC: area under the curve; IVUS: intravascular ultrasound; OC: overlapped calcification; OFDI: optical frequency domain imaging
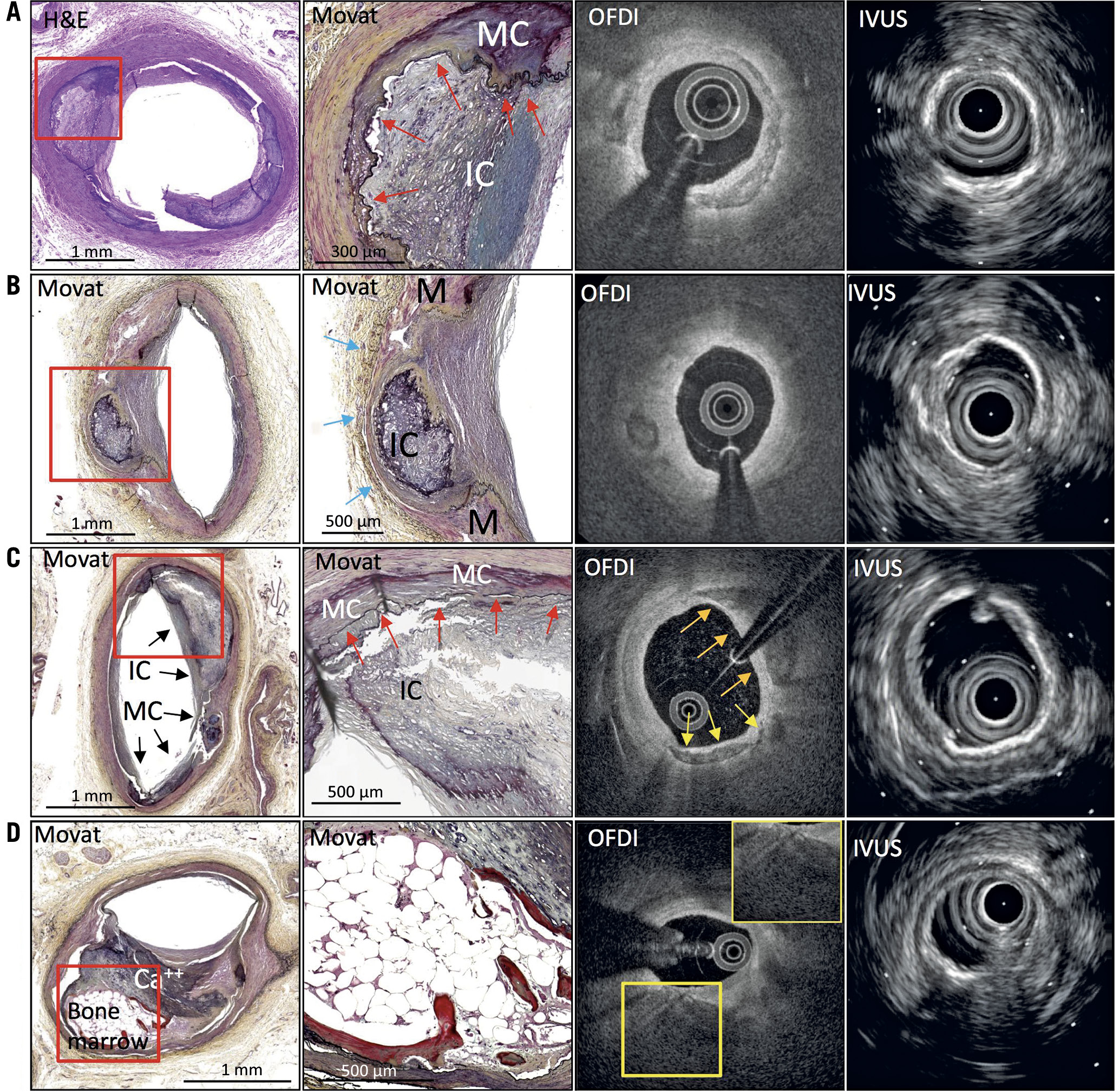
Figure 5. Misreading cases of medial calcification and bone formation. The left panels in histology show low-power images and the adjacent panels show high-power images from the red boxes. A) Fusion calcification. Intimal (IC) and medial calcification (MC) are connected over internal elastic lamina (red arrows). OFDI shows calcification in intima and IVUS shows high echoic signal with acoustic shadow within the intima. B) Medial thinning from intimal calcification. Intimal calcification invades the medial wall where the media (M) becomes thin and is almost absent (blue arrows). OFDI shows calcification located deep in the intima, and IVUS shows high echoic signal near the intima-media border. C) Intimal and medial calcifications are located side by side. The high-power images show that the IC and MC are connected over internal elastic lamina (red arrows). OFDI shows likely intimal calcification (orange arrows) and medial calcification (yellow arrows). IVUS shows likely medial calcification. D) Histological image shows bone formation with overlying thick calcification (Ca++). OFDI shows thick calcification. It is difficult to observe the deeper area due to attenuation from thick calcification. IVUS shows high echoic signal at the luminal surface. IVUS: intravascular ultrasound; OFDI: optical frequency domain imaging
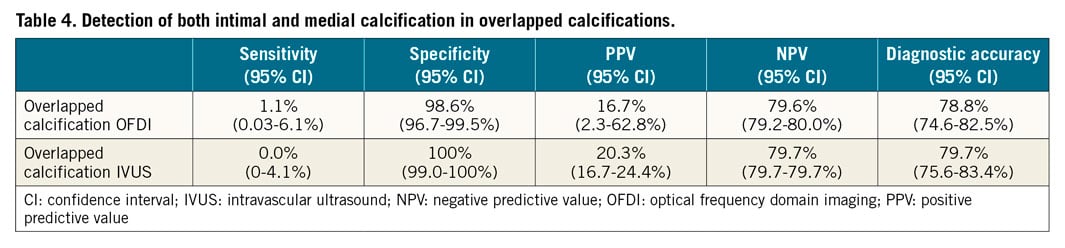
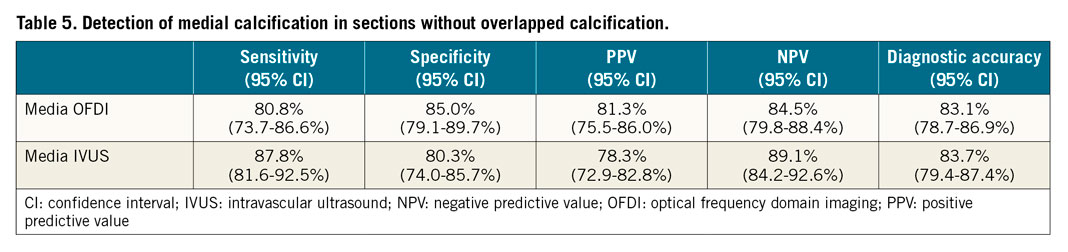
Detection of the presence of both media and intima in overlapped calcification by OFDI was poor (sensitivity 1.1%) and impossible by IVUS (sensitivity 0.0%) (Table 4, Supplementary Table 3). Of the 349 sections, 89 had overlapped calcifications; detection of medial calcification by OFDI and IVUS was improved if the overlapped sections were excluded from analysis (sensitivity/specificity/AUC, OFDI: 81%/85%/0.83 and IVUS: 88%/80%/0.84, respectively) (Figure 4, Table 5, Supplementary Table 4, Supplementary Figure 2).
DIAGNOSTIC CAPABILITY FOR BONE FORMATION BY OFDI
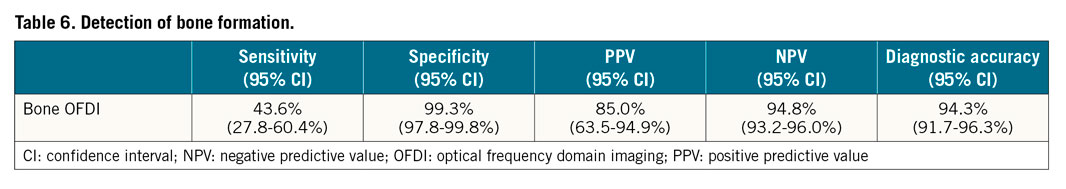
Bone formation by OFDI has unique findings, e.g., honeycomb sign; however, IVUS showed only a strong high echoic sign due to overlying calcification or the presence of the trabecular bone. Therefore, IVUS could not distinguish bone formation from other types of calcification. Of the 39 with bone formation by histology, 32 were found in intima and 7 were in media. Of 22 lesions which were not diagnosed as bone formation, 15 were in intima and 7 were in media (sensitivity 43.6%, and specificity 99.3%) (Table 6, Supplementary Table 5). Three were misdiagnosed as bone formation because of a high-intensity signal inside calcification mimicking honeycomb lesions (PPV 85.0% and NPV 94.8%). The main reason (in 10 [46%] of 22) for this was overlying calcification, followed by absent or small areas of bone marrow (9 [41%], signal attenuation (2 [0.9%]), and a deep location (1 [0.5%]) (Figure 5).
MEASUREMENT OF AN AREA AND ARC OF CALCIFICATION BY OFDI AND IVUS
Linear regression showed a poorer correlation of area of calcification between OFDI and histology in ATK than in BTK (R2, ATK 0.60 with p<0.001, BTK 0.76 with p<0.001) (Supplementary Figure 3). Also, a poorer correlation of arc of calcification between OFDI and histology was shown in ATK by OFDI than in BTK (R2, ATK 0.63 with p<0.001, BTK 0.89 with p<0.001). There was a better correlation between IVUS and histology in both ATK and BTK (R2, ATK 0.86 with p<0.001, BTK 0.82 with p<0.001) (Supplementary Figure 3).
Discussion
Our main findings are as follows. 1) Medial calcification was less detectable by both OFDI and IVUS than intimal calcification due to the presence of overlapped (medial and intimal) calcification and inability to distinguish clearly between intima and media. 2) In the absence of such overlapped calcification, the detection of medial calcification was improved. 3) OFDI bone formation was defined for the first time as a honeycomb lesion in contact with calcification, a definition which showed high specificity with low sensitivity due mainly to overlying calcification. IVUS was extremely poor at detecting bone formation due to acoustic shadows derived from overlying calcification. 4) The area and arc of measured calcification had a poorer correlation between OFDI and histology in ATK than in BTK territories. On the other hand, those measurements by IVUS showed an excellent correlation with histology for both ATK and BTK.
IMPORTANCE TO DISTINGUISH MEDIAL FROM INTIMAL CALCIFICATION
Medial calcification is associated with worse cardiovascular mobility and higher mortality7. Medial calcification can lead to an increase in vessel stiffness8. In the advanced stage, a loss of elasticity decreases peripheral tissue perfusion, leading to flow stasis and diffuse thrombus formation9. However, the prevalence of medial calcification in PAD is imprecisely reported due to methodology, lack of clear association with symptoms, and limitations of imaging technologies4. Also, in a recent work using IVUS to validate three different angiographic calcium scoring systems, both medial and intimal calcification were categorised together based on distribution, regardless of the type of calcification. However, we believe the type of calcification should also play a role in determining strategies for EVT4.
Considering its thickness and distribution and the fact that it does not generally cause luminal stenosis, aggressive high pressure by balloon angioplasty for medial calcification is typically not warranted. When present, care should be taken in avoiding high-pressure dilatation for treatment of lesions associated with medial calcification because the risk of vascular perforation and medial dissection can be minimised by doing so. Excessive high tensile stress is generated at the junction between tissue types with differing elastic properties and thus delivering high pressure to medial calcifications might lead to medial dissection as well as fracture of medial calcification10. Medial dissection may occur at the junction between normal and calcified media and result in excessive growth of neointima11. In a study using rats, Marshall et al reported that neointimal hyperplasia was significantly increased in the carotid arteries with medial calcification after balloon catheter injury when compared to the controls12. It was suggested that medial calcification itself induced neointimal hyperplasia after balloon angioplasty.
Different atherectomy techniques are currently in clinical use such as rotational and orbital atherectomy as well as directional atherectomy13. In general, medial calcification is not the target of such techniques and the inability to distinguish intimal from medial calcification might lead to vascular perforation and medial injury for the reasons mentioned above14. Therefore, interventions directed solely at medial calcifications should be minimised, and even when both intimal and medial calcification coexist in the same lesion, extra care should be taken to avoid excessive medial injury.
DETECTION OF MEDIAL CALCIFICATION
To the best of our knowledge, this study is the first to validate the detection of medial and intimal calcification. Also, the definition of medial calcification by IVUS has never been described well. In a review of the literature, no acoustic shadows due to the presence of fibrous tissue have been described by IVUS as a typical finding of medial calcification9. However, only calcified IEL, or medial calcification when it is close to IEL, typically shows no acoustic shadows, whereas medial calcification deep in the media should show acoustic shadow such as intimal calcification regardless of the proportion of fibrous tissue. However, when excluding overlapped calcification as a main cause of the less reliable detection of medial calcification, the detection of medial calcification is improved. For decisions regarding strategy for endovascular treatment, determining whether calcification is present on the luminal side is of primary importance.
BONE FORMATION
In PAD, bone formation is not rare. The incidence of bone formation has been reported to be 19-83% in patients with or without leg amputation1,15. Bone formation can be observed in heavily calcified lesions and can be considered the most advanced stage of calcification2,9. Severe calcification is seen as a feature of stable plaques by pathological analysis2. Plaques with bone formation, which is typically associated with severe calcification, can probably be considered to be locally stable plaque, although overall these patients may have a high risk of clinical events due to the many conditions which are comorbid with such features2. Findings of bone formation in arteries by intravascular imaging devices have not been described. Bone marrow in bone can be detected as a honeycomb sign by OFDI, although mainly overlying calcification can make bone invisible due to attenuation. Detection of bone formation in PAD may help to provide a better understanding of risk stratification and local plaque stability.
Limitations
There are several limitations in this study. First, data by 60 mHz-IVUS cannot be applied to 20- or 40 mHz-IVUS. Second, there are no clear data showing clinical outcomes of patients with medial calcification and bone formation in peripheral arteries. Therefore, further investigation is needed to assess clinical outcomes in patients with these findings. Finally, findings of IVUS and OFDI in an ex vivo setting might be different from those in an in vivo setting.
Conclusions
Medial calcification was less detectable by OFDI and IVUS than intimal calcification, especially in the presence of overlapped calcification where there was an inability to clearly distinguish the border between intima and media. Without overlapped calcification, the detection of medial calcification was improved and acceptable. Detection of bone formation by OFDI provided high specificity with low sensitivity.
|
Impact on daily practice In general, medial calcification alone does not require an aggressive strategy such as high-pressure balloon dilatation and/or atherectomy devices since medial calcification is typically thin and fragile. Using such aggressive approaches on medial calcification can lead to vascular perforation and injury which results in additional vascular trauma and restenosis. Therefore, the distinction between medial and intimal calcification by intravascular imaging may decrease unnecessary use of these approaches and also reduce vascular complications. |
Acknowledgements
We thank Xin Xu for her professional work.
Funding
This study was funded by Terumo, Tokyo, Japan. CVPath Institute, Inc., Gaithersburg, MD, USA, provided full support for this work.
Conflict of interest statement
R. Virmani and A.V. Finn have received institutional research support from Abbott Vascular, Boston Scientific, and Terumo Corporation. A. Finn has received honoraria from Abbott Vascular, Boston Scientific, and Terumo Corporation, and is a consultant to Abbott Vascular, and Boston Scientific. R. Virmani has received honoraria from Abbott Vascular, Boston Scientific, and Terumo Corporation, and is a consultant to Abbott Vascular, Boston Scientific, and Terumo Corporation. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

