We live in extraordinary times. The current COVID crisis has understandably overshadowed developments in cardiovascular medicine over the last few months. However, it is important to reflect that, even at the COVID peak, daily deaths from COVID-19 and cardiovascular disease were 7,504 and 48,742, respectively.
During European lockdown, the results of the ISCHEMIA trial were published1 and an opportunity to pore over the supplementary data of this landmark trial was made available to all. Contemporary optimal medical therapy (OMT) demonstrated equivalence to an invasive strategy in a stable cohort of patients with symptoms of angina and evidence of moderate to severe ischaemia. Pre-randomisation blinded computed tomography coronary angiography (CTCA) highlighted three-vessel coronary disease with ≥50% stenosis in 45% and involvement of the proximal left anterior descending artery in 47% of all patients. In this context it is unsurprising that the revascularisation rate in the invasive arm of the study exceeded 80%. However, the subsequent finding of equipoise with a conservative strategy, that only necessitated revascularisation for ischaemic events or uncontrolled symptoms, in approximately 20% of patients, questions our existing reliance on ischaemia and stenosis identification to guide treatment. Furthermore, unpublished data presented by the study authors have demonstrated plaque burden (number of diseased vessels) as being more predictive of subsequent events than the extent of ischaemia. Consequently, it is likely that we observe an increasing reliance on anatomical/morphological assessment in the investigation and guidance of treatment for patients with chronic coronary syndrome. However, it is important to reflect that, regardless of the strategy adopted and despite OMT, more than 10% of the study population sustained a myocardial infarction after five years. This observation leads us to question how best we can identify patients or plaques that are vulnerable to future events. As pathways evolve for the identification and treatment of patients with chronic coronary syndromes, our major priority must be an avoidance of acute coronary events and protection against cardiovascular death. The existing focus on ischaemic and anatomical assessment may be insufficient. In addition to plaque burden/distribution and ischaemic potential, we should consider plaque vulnerability to guide decision making and treatment escalation (Figure 1).
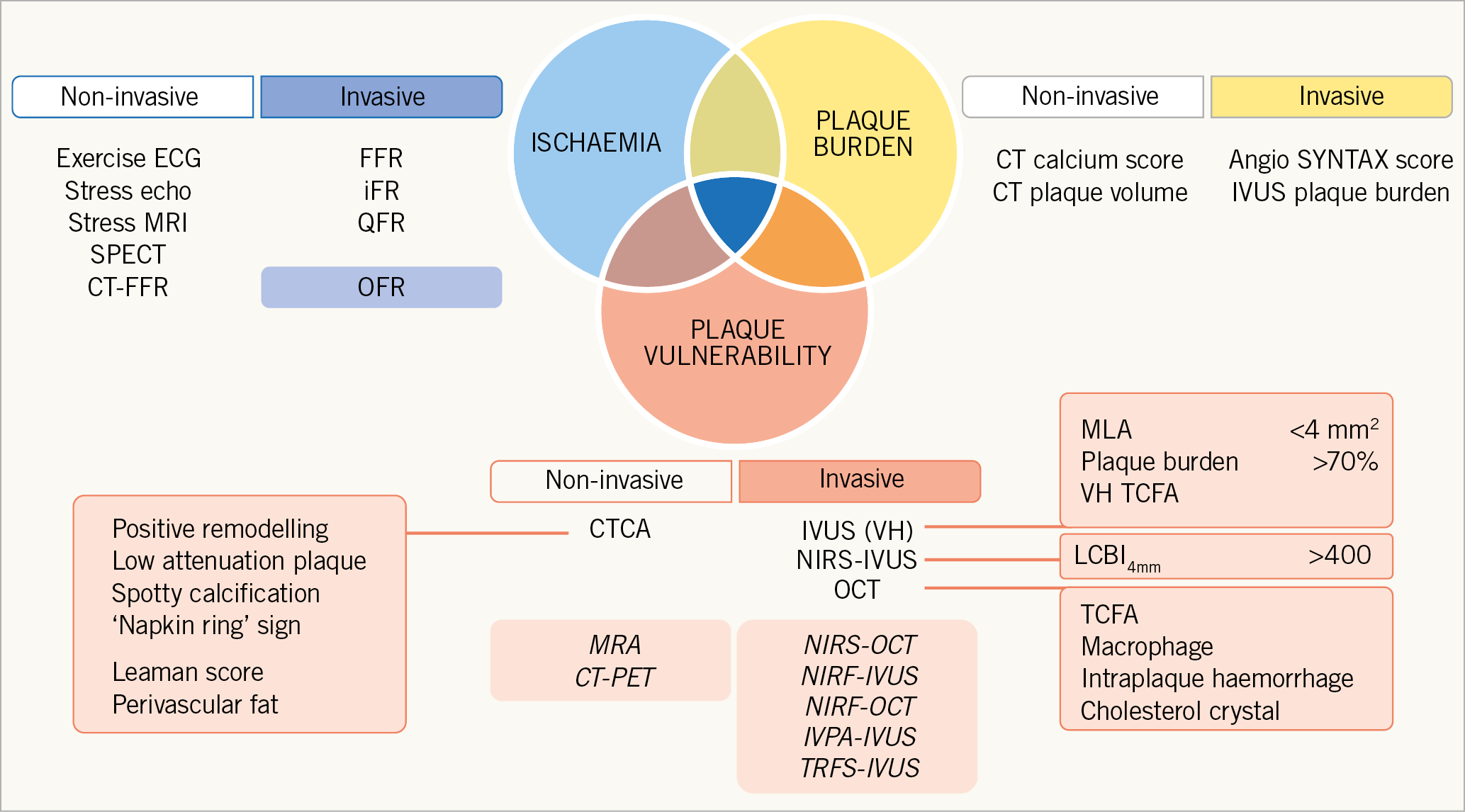
Figure 1. Seeking the “Holy Grail” of coronary artery disease identification and risk stratification. Modalities and markers predominantly of academic interest are shaded.
The concept of plaque vulnerability has been in existence since the 1980s. However, the impact of defining features of vulnerability has been limited by the relatively low positive predictive value of current imaging modalities to predict major adverse cardiac events (MACE)2. Clearly, non-invasive imaging, particularly CTCA, provides significant advantages for large-scale surveillance of “at risk” populations and with short acquisition times can accurately delineate the presence or absence of coronary disease with high sensitivity and specificity3. However, with a spatial resolution of 0.5 mm, identification of specific markers of vulnerability is limited. Low-attenuation plaque has been shown to be associated with increased risk. Most recently, a substudy of the SCOT-HEART trial demonstrated that patients with a low-attenuation plaque burden >4% had an almost fivefold risk of sustaining a myocardial infarction4. Combined positron emission tomography (PET)-CT provides future potential for identification of vulnerability markers including inflammation5. Greatest interest has been directed towards invasive methods of identification of markers of plaque vulnerability. Intravascular ultrasound (IVUS) has identified large plaque burden, small lumen area and, by virtual histology, presence of thin-cap fibroatheroma (TCFA) as markers of vulnerability. However, as with CT, limitations of resolution (100 um) have been flagged as the reason for limited predictive value of future events. Recently, the combination of IVUS with near-infrared spectroscopy (NIRS-IVUS) has demonstrated an ability to identify non-culprit lipid-rich plaques at risk of MACE, independently of plaque burden or minimum lumen area6. Optical frequency domain imaging (OFDI)/optical coherence tomography (OCT) provides tenfold higher resolution, aiding detection of TCFA, and is currently being tested alongside IVUS and NIRS in a 1,600-patient trial of preventative therapy in patients with features of vulnerability (PREVENT: NCT02316886).
Current study
The team from the CVPath Institute has led the way in defining coronary plaque types by histology. Their ex vivo comparison of OFDI/OCT of human coronary arteries with histology has been essential in developing our understanding and ability to interpret in vivo imaging in our patients. The current study has focused upon the detection of cholesterol crystals, a marker of advanced plaque, associated with necrotic core formation7.
Until now, cholesterol crystals have been defined exclusively as “high linear signal within the plaque”. Jinnouchi and colleagues redefine the imaging signature of cholesterol crystals as a high-intensity, linear signal (bright area) within the plaque, distinguished from macrophage by the presence of a sharp border, distinct from surrounding low- or moderate-intensity areas, unrelated to areas of calcification. Meticulous co-registration of histology and ex vivo imaging enabled confirmation of image-based detection of cholesterol crystals with a positive predictive value of 100%. However, the complexity of plaque and associated limitations of OFDI/OCT imaging obscured detection of all cholesterol crystals, with a sensitivity of 25.6%. Features favouring OFDI/OCT detection included shallow depth, stacking of cholesterol clefts at 90° to the imaging angle and overlying fibrotic tissue. Conversely, complex tissue components, including highly attenuating foam cells and overlying calcification obscured detection of cholesterol crystals by intravascular imaging. Despite these limitations, the development of a more detailed definition of cholesterol crystals avoided misinterpretation in more than 70% of plaque cross-sections (commonly foam-cell macrophage or associated with calcification).
Tissue characterisation is challenging and, even in expert hands, misidentification of plaque type will occur. In this study, the classic signature of calcium (a well delineated area of low signal) enabled consistent identification of fibrocalcific plaque (170/180 plaques). Similarly, the presence of rupture (4/5 plaques) was well observed but thin-capped fibroatheroma, the archetypal “vulnerable plaque”, was harder to identify (9/15 plaques). Lipid-rich plaque was frequently observed, with inclusion of over 100 early plaques limited to pathological intimal thickening. The authors highlight the potential added benefit of identifying cholesterol crystals as a marker of plaque maturity, with the presence of cholesterol crystals almost exclusively observed within advanced plaque morphology. Therefore, it is hoped that OFDI/OCT may provide more accurate identification of necrotic core plaque, aiding risk stratification and tailored intensification of therapy to avoid future MACE.
Final remarks
This study highlights the best and worst of intravascular imaging, demonstrating a significant gap between identification of plaque vulnerability and clinical reality. OFDI/OCT is an advanced technology providing incredible resolution and in expert hands an ability to delineate high-risk markers of vulnerability. However, the hostile environment of the diseased coronary artery and specific characteristics of mature plaque components, including thrombus, foam-cell macrophage and lipid will continue to limit our ability to characterise plaque accurately. Additionally, we must acknowledge that pressure of time, money and a lack of education will continue to inhibit the adoption and use of intravascular imaging in routine clinical practice8. The technical challenges of identifying markers of vulnerability, irrespective of imaging modality, should be overcome with the aid of machine learning9. With the evolution of patient pathways and an increasing utilisation of multimodality plaque imaging, we should look to harness as much information as possible to enhance our ability to risk stratify patients appropriately and target treatment intensification to those at greatest risk.
Conflict of interest statement
T. Johnson has received honoraria or consultation fees from Abbott, Bayer AG, Biosensors, Boston Scientific, Medtronic, Terumo and Vascular Perspectives; received grants/research support from AstraZeneca and Bayer; and participates in a company sponsored speaker’s bureau for Abbott. N. Joshi has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.