Abstract
Aims: The aim of this study was to determine the ability of optical frequency domain imaging (OFDI)/optical coherence tomography (OCT) imaging systems to visualise the presence of cholesterol crystals (CCR) in human atherosclerotic coronary arteries.
Methods and results: We performed ex vivo imaging of human coronary arteries by OFDI/OCT. A total of 559 cross-sectional images from 45 autopsy cases were co-registered with histology; 117 histologic sections showed presence of necrotic core with cholesterol clefts (CC). We modified a previously used OFDI/OCT definition for identification of CCRs which we now define as a linear and discrete high-intensity signal (bright area) within the plaque with sharp borders between it and adjacent low-/intermediate-intensity tissue. Additionally, the high-intensity signal is not a spot but a well-defined area distinguishing it from macrophages which lack sharp borders. OFDI/OCT imaging identified the presence of CCR in 30 of the 117 histologic sections. The sensitivity and specificity of OFDI/OCT for detection of CCR was 25.6% and 100.0%, respectively. By multivariate analysis, significant predictors to visualise CCR by OCT/OFDI were 1) an overlying fibrous plaque, and 2) the presence of stacked CC, defined as CC arranged one on top of another with >3 layers of CC. The prevalence of complicated plaques (i.e., plaque haemorrhage and late necrotic core) was significantly higher in detectable CCR by OFDI/OCT as compared to undetectable CCR.
Conclusions: The presence of stacked CCs is required to detect CCR by OFDI/OCT. Detection of CCR by OCT/OFDI may help us to identify the late stages of atherosclerotic coronary plaque progression and improve risk stratification.
Introduction
Atherosclerotic coronary artery disease is the leading cause of death worldwide. Many studies have reported on the mechanisms of progression of atherosclerotic lesions. Atherosclerotic plaques develop through the deposition of lipid in acellular areas of the intima which are rich in proteoglycans, and form what is known as a lipid pool. Lipid pools are mostly devoid of cholesterol crystals (CCR) but progress to form necrotic cores by macrophage infiltration and subsequent phagocytosis of lipid which causes them eventually to undergo apoptosis, forming acellular debris with the release of free cholesterol1. Free cholesterol has also been shown to be derived from red cell membranes that are observed as plaques thicken and vasa vasorum rupture leading to intraplaque haemorrhages. Plaque rupture is the most frequent cause of acute coronary syndromes1. Necrotic cores are composed of acellular debris rich in CCR which are formed by the crystallisation of free cholesterol. Recent studies have shown that CCR trigger inflammation by the release inflammasomes which facilitate the development of lipid-rich necrotic core2. The presence of CCR is associated with worse clinical outcomes through distal embolisation in patients receiving percutaneous coronary intervention3. Therefore, the presence of CCR has been considered to be an indicator of advanced plaque.
Diagnostic modalities that have the ability to identify plaque composition could be used to improve risk stratification and to guide therapy to prevent untoward cardiovascular events. Optical frequency domain imaging (OFDI) and optical coherence tomography (OCT) imaging are high-resolution intravascular imaging modalities that provide a maximal axial resolution of 10-20 µm, allowing identification of tissue characteristics. However, not all CCR are detectable by OFDI/OCT. The reason why CCR are detectable in some plaques and not in others remains unclear. In addition, the pathophysiological significance of the presence of detectable CCR is unknown. To the best of our knowledge, there are no studies examining the accuracy of OFDI/OCT for detection of CCR. Our study sought to evaluate whether CCR could be identified by OFDI/OCT (with histology as the gold standard) and to determine why some CCR are detectable by OFDI/OCT while others remain undetectable in human coronary arteries.
Methods
STUDY POPULATION
A total of 55 vessels from 45 patients underwent OFDI or OCT with corresponding histologic sections and were included in the study. Supplementary Appendix 1 provides more details.
CO-REGISTRATION OF OFDI/OCT IMAGES WITH HISTOLOGY
All OFDI and OCT images were co-registered with histologic sections by an experienced investigator (H. Jinnouchi). For careful co-registration between OFDI/OCT images and histological sections, side branches and the distance from the nearest ostium were taken into consideration, and anatomical landmarks such as calcification and luminal configuration were considered to adjust images longitudinally and circumferentially.
CLASSIFICATION OF ATHEROSCLEROTIC PLAQUES IN HISTOLOGY
All histology slides were reviewed by experienced readers (H. Jinnouchi and R. Virmani). Coronary plaques were classified using the modified American Heart Association classification: 1) adaptive intimal thickening (AIT) or fibrous plaque (FIB), defined as lesions with predominantly fibrous tissue and no lipid pool; 2) pathological intimal thickening (PIT), defined as lesions with lipid pools but absence of necrosis; 3) fibroatheroma (FA), defined as plaques containing necrotic core (±plaque haemorrhage) with thick fibrous cap thickness (>65 µm); 4) thin-cap fibroatheroma (TCFA), defined as FA with the thinnest portion of the fibrous cap ≤65 µm in thickness and the cap infiltrated by macrophages and lymphocytes with an underlying necrotic core (±plaque haemorrhage); 5) fibrocalcific plaque, defined as lesions with severe calcification with absence or only a small fraction of necrotic core; and 6) plaque rupture with a disrupted fibrous cap and an overlying thrombus. FA was further divided into early and late FA dependent on the presence or absence of a matrix (proteoglycan or collagen)1. Early FA was defined as plaques with necrotic core in which focal free cholesterol may be present with focal loss of proteoglycans and/or collagen with macrophage infiltration, whereas late FA was defined as plaque with necrotic core and nearly complete depletion of the extracellular matrix with increase in free cholesterol clefts (CC). In addition, all plaques with necrotic cores were classified as early and late necrotic core, including FA, TCFA and plaque rupture. The overlying plaque (above the necrotic core) was also assessed for presence of fibrous tissue, presence or absence of high density of foamy macrophages, calcification, PIT lesion and overlying thrombus. Stacked CC were defined as multiple large CC overlying one another with greater than three layers of CC within areas of necrotic core (Figure 1).
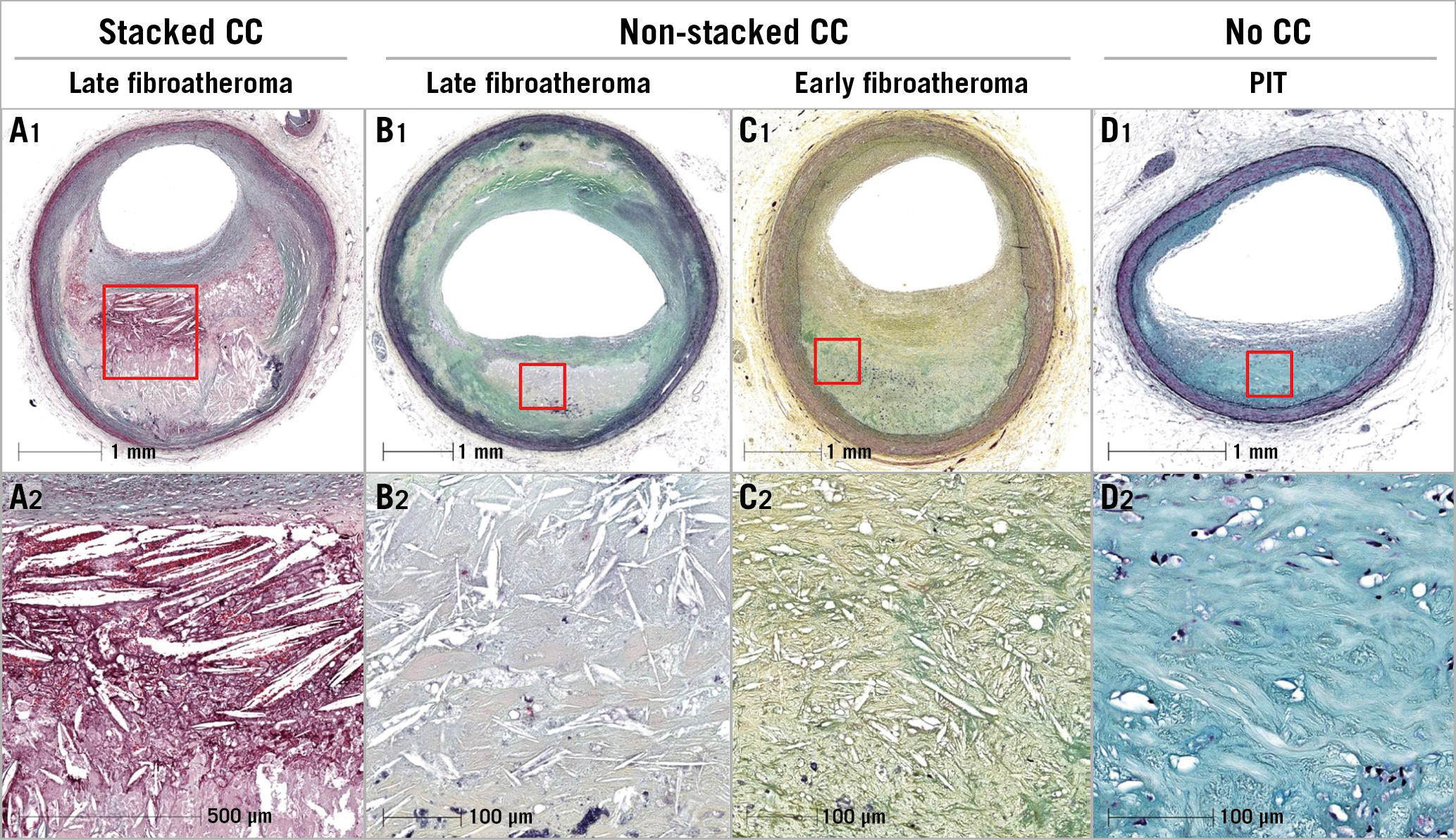
Figure 1. Different types of cholesterol clefts (CC). A1) & A2) Low- and high-power images showing late necrotic core, stacked CC, haemorrhage, overlying fibrous tissue and severe luminal stenosis. B1) & B2) Low- and high-power images showing late fibroatheroma with small CC and moderate coronary stenosis. C1) & C2) Low- and high-power images showing early fibroatheroma, moderate luminal stenosis, thick fibrous cap and small CC. D1) & D2) Low- and high-power images showing pathological intimal thickening (PIT) lesion, lipid pool with absence of CC, and <50% luminal stenosis.
OFDI AND OCT IMAGING ANALYSIS
Two OFDI/OCT readers (Y. Sato and A. Sakamoto) blinded to the histologic findings assessed OFDI/OCT images independently. The prior definition of CCR as a “high linear signal within the plaque” has been broadly used by multiple investigators4,5 but lacks specific detail. We defined CCR using the following criteria: 1) a linear, discrete, high-intensity signal (bright area) within the plaque; 2) a clear border must exist between this signal and adjacent low- or intermediate-intensity tissue; 3) the linear high-intensity signal is not a spot but a well-defined area distinguishing it from macrophages which lack sharp borders; and 4) it is not located at borders of calcification or within areas of calcification. When the diagnosis was inconsistent between the two readers, a consensus was achieved through discussion. Quantitative OFDI/OCT measurements were performed using a planimetry software (QIvus® 3.0; Medis medical imaging systems, Leiden, the Netherlands) (Figure 2). Catheter-CCR angle was defined as the angle between the longitudinal line of the CCR and a line drawn from the centre of a catheter light sensor to the middle of the CCR. Depth of CCR was defined as the distance from the luminal surface to the middle of the CCR. Catheter-CCR distance was defined as the length from the centre of the catheter to the centre of the CCR.
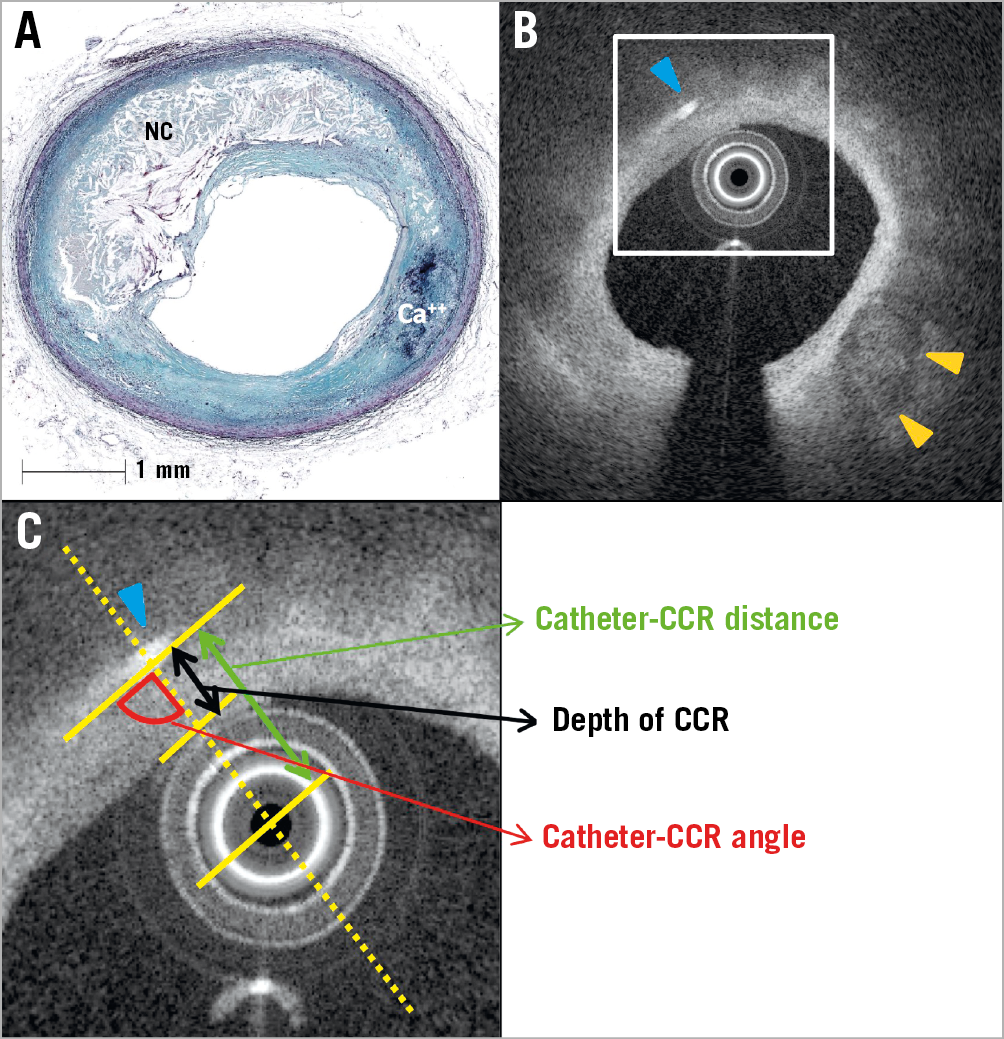
Figure 2. Methods to evaluate OFDI/OCT-derived CCR. A) Histological image showing a fibroatheroma lesion with a necrotic core (Movat pentachrome stain). B) Co-registered OFDI cross-sectional image showing the presence of CCR (blue arrowhead) and calcification (orange arrowheads). C) High-power image of the white box from image B. Catheter-CCR distance is the distance between the centre of the sensor catheter tip and CCR (green double arrow); depth of CCR is the distance from the luminal surface of the catheter to the middle of CCR (black double arrow); and catheter-CCR angle is the angle between the longitudinal line of the CCR and the line from the centre of the catheter to the middle of CCR (red quarter-circle). Ca++: calcification; CCR: cholesterol crystals; NC: necrotic core; OCT: optical coherence tomography; OFDI: optical frequency domain imaging
STATISTICAL ANALYSIS
Details of the statistical analysis can be found in Supplementary Appendix 1.
Results
CLINICAL AND MORPHOLOGIC INFORMATION
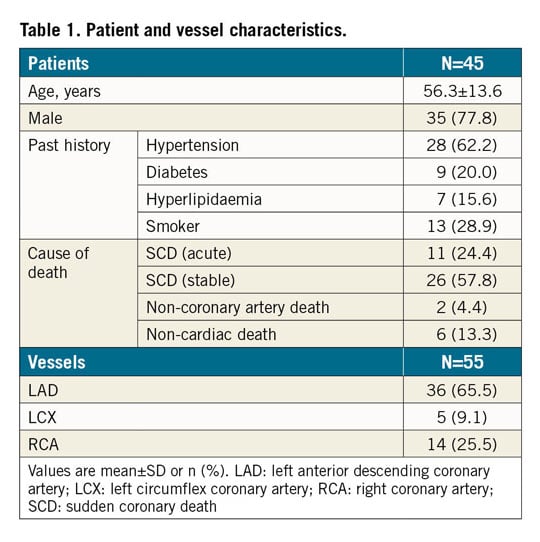
Clinical characteristics of the vessels that had undergone OFDI or OCT are listed in Table 1. A total of 573 histologic cross-sections were available for histologic assessment and co-registration. Sections with processing artefact (n=4), post-mortem clot (n=4) and poor images (n=6) were not included in the analysis. A total of 559 images were co-registered with OFDI/OCT images for further analysis.
HISTOLOGIC ASSESSMENT OF THE TYPE OF PLAQUE AND PRESENCE OF CCR
Each histologic section (n=559) was classified by the type of plaque morphology: AIT/FIB 108 (19.3%), PIT 154 (27.5%), early FA 29 (5.2%), late FA 62 (11.1%), TCFA 15 (2.7%), plaque rupture 5 (0.9%), fibrocalcific (FC) + necrotic core 6 (1.1%), and FC 180 (32.2%) sections, respectively. A total of 117 sections showed presence of necrotic core (Supplementary Table 1) and all had histologic evidence of CC (Figure 3). However, by OFDI/OCT, only 30 (25.6%) frames showed presence of CCR and all were also identified by histology with a positive predictive value (PPV) of 100.0%, negative predictive value (NPV) of 83.6%, with a sensitivity of 25.6%, specificity of 100.0% and accuracy of 84.4% (Table 2, Table 3). Of 30 OFDI/OCT images showing CCR, the majority (86.7%) showed presence of stacked CC by histology. Stacked CC by histology were identified in 45 of 117 (38.5%) cases with necrotic core. The PPV, NPV, sensitivity, specificity and accuracy for detection of stacked CC by OCT/OFDI were 86.7%, 96.4%, 57.8%, 99.2% and 95.9%, respectively. Figure 4 and Supplementary Figure 1 show representative early and late FA, plaque haemorrhage, TCFA and rupture and corresponding OFDI/OCT images. Supplementary Appendix 2 and Supplementary Table 2 show aspects of the analysis.
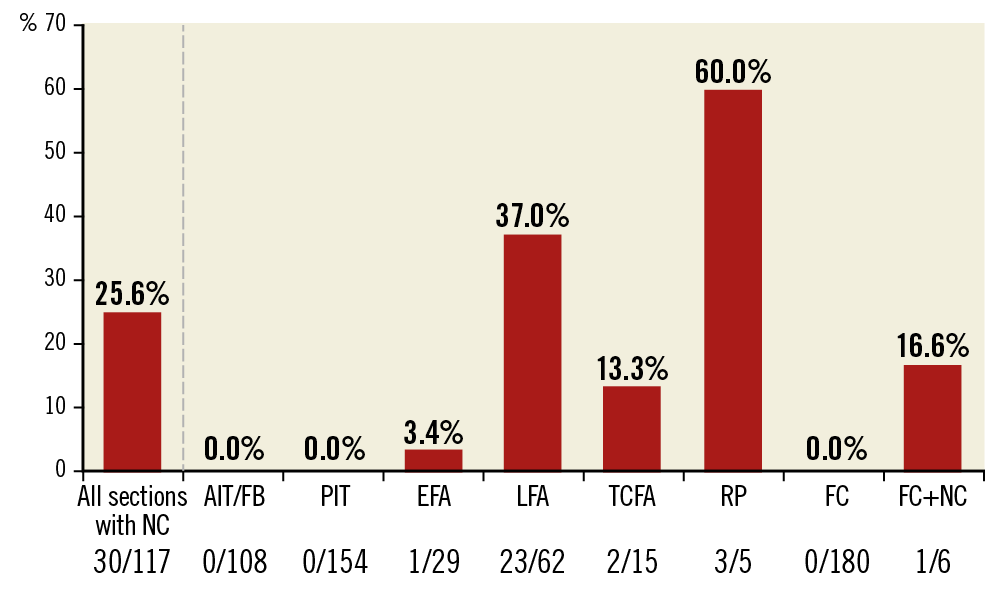
Figure 3. Bar graph showing the distribution of CCR detectable by OFDI/OCT based on type of plaque. Plaque type was classified using histological images. Data are expressed as the total number of frames with CCR detected by OFDI/OCT over the total number of sections examined histologically stratified by plaque type. AIT: adaptive intimal thickening; CCR: cholesterol crystals; EFA: early fibroatheroma; FC: fibrocalcific plaque; FIB: fibrous plaque; LFA: late fibroatheroma; NC: necrotic core; OCT: optical coherence tomography; OFDI: optical frequency domain imaging; PIT: pathological intimal thickening; RP: plaque rupture; TCFA: thin-cap fibroatheroma
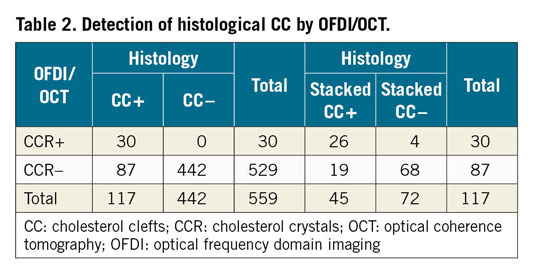
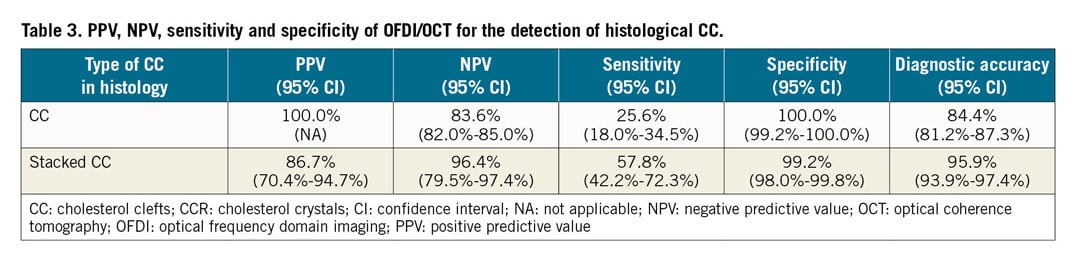
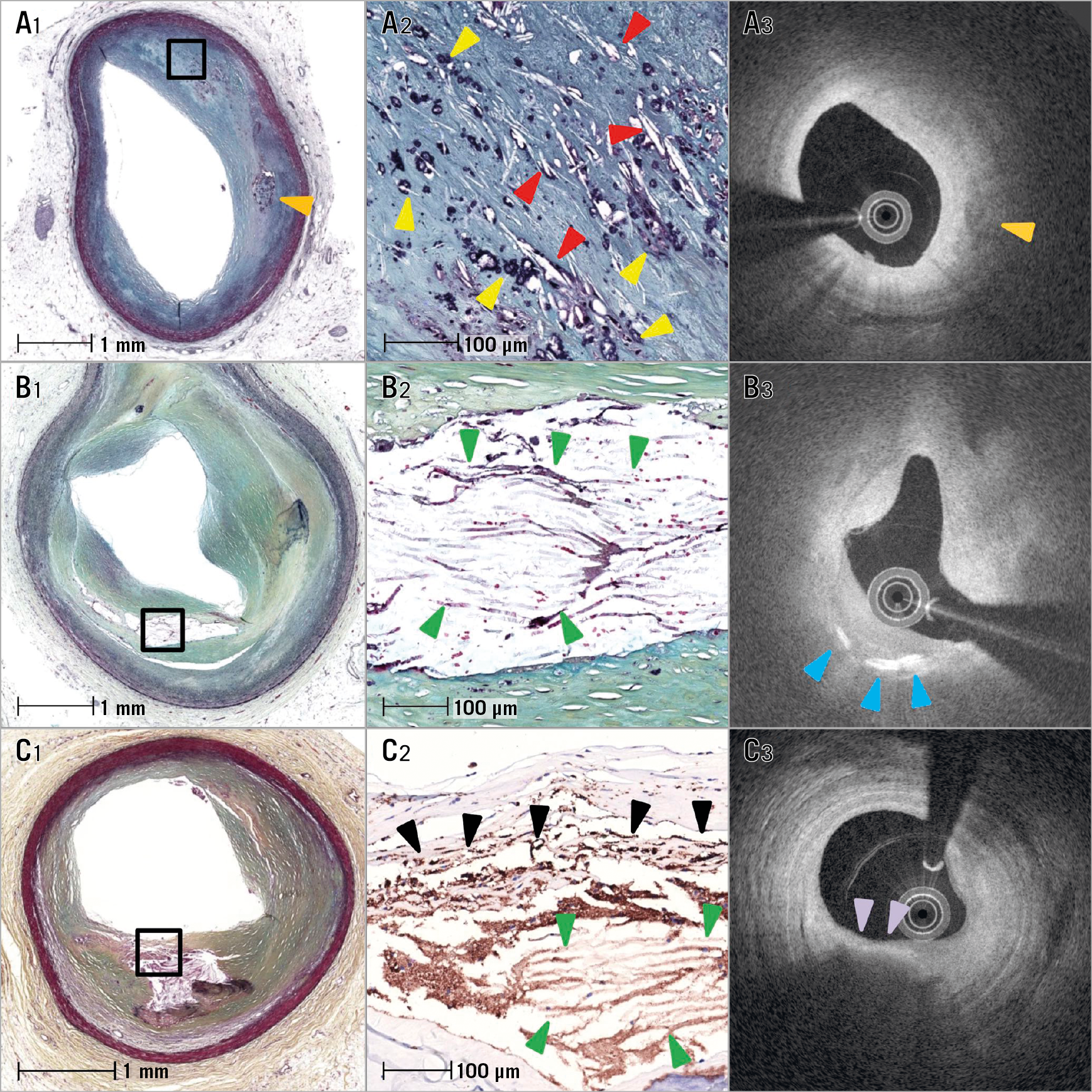
Figure 4. Histological images of early and late fibroatheroma with corresponding OFDI images. A1) Low-power image showing early fibroatheroma with focal necrosis within the lipid pool, macrophage infiltration and calcification (orange arrowhead). A2) High-power image from A1 showing early necrotic core with interspersed CCR (red arrowheads) and punctate calcifications (yellow arrowheads) of macrophages. A3) OFDI image showing low-intensity area with absence of CCR and calcification (orange arrowhead) in the lipid pool. B1) & B2) Low- and corresponding high-power images of the boxed area showing a late fibroatheroma with a necrotic core area filled with stacked CC (green arrowheads). B3) OFDI image showing CCR (blue arrowheads). C1) & C2) Low- and high-power images showing a late fibroatheroma with stacked CC (green arrowheads) and overlying foamy macrophages (black arrowheads). High-power image (C2) is macrophage (CD68) stained image from the box in C1. C3) Corresponding OFDI image showing macrophages with attenuation (purple arrowheads) and no CCR. All histologic images are Movat stained except for C2 (CD68 staining). CC: cholesterol clefts; CCR: cholesterol crystals; OCT: optical coherence tomography; OFDI: optical frequency domain imaging
COMPARISON OF DETECTABLE VERSUS UNDETECTABLE CCR BY OFDI/OCT TO THE MORPHOLOGIC FINDINGS
Of 117 sections with necrotic core by histology, 30 sections had detectable CCR and 87 sections no CCR by OFDI/OCT. Both groups underwent histologic measurements (Table 4). In terms of basic morphometry (external elastic lamina [EEL], internal elastic lamina [IEL], lumen area and percent stenosis), there were no significant differences between the two groups. The tissue overlying (i.e., above) the CCR was assessed and showed significant differences between plaques with detectable and undetectable CCR, with more frequent fibrous tissue, and fewer foam cells and less thrombus in detectable CCR (Figure 5A). Detectable CCR also showed a significantly higher prevalence of late necrotic core as compared to undetectable CCR. The presence of haemorrhage within the necrotic core was significantly more frequent in detectable CCR as compared to undetectable CCR. The length of the CC was also significantly greater in detectable versus non-detectable CCR. Detectable CCR showed significantly greater thickness of CC than undetectable.
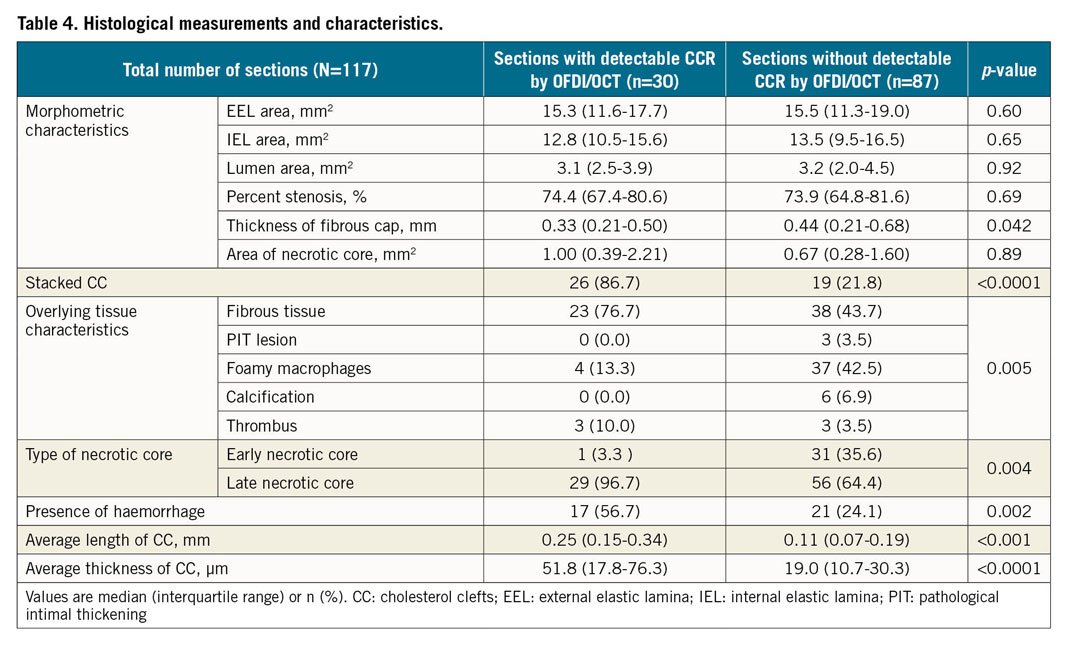
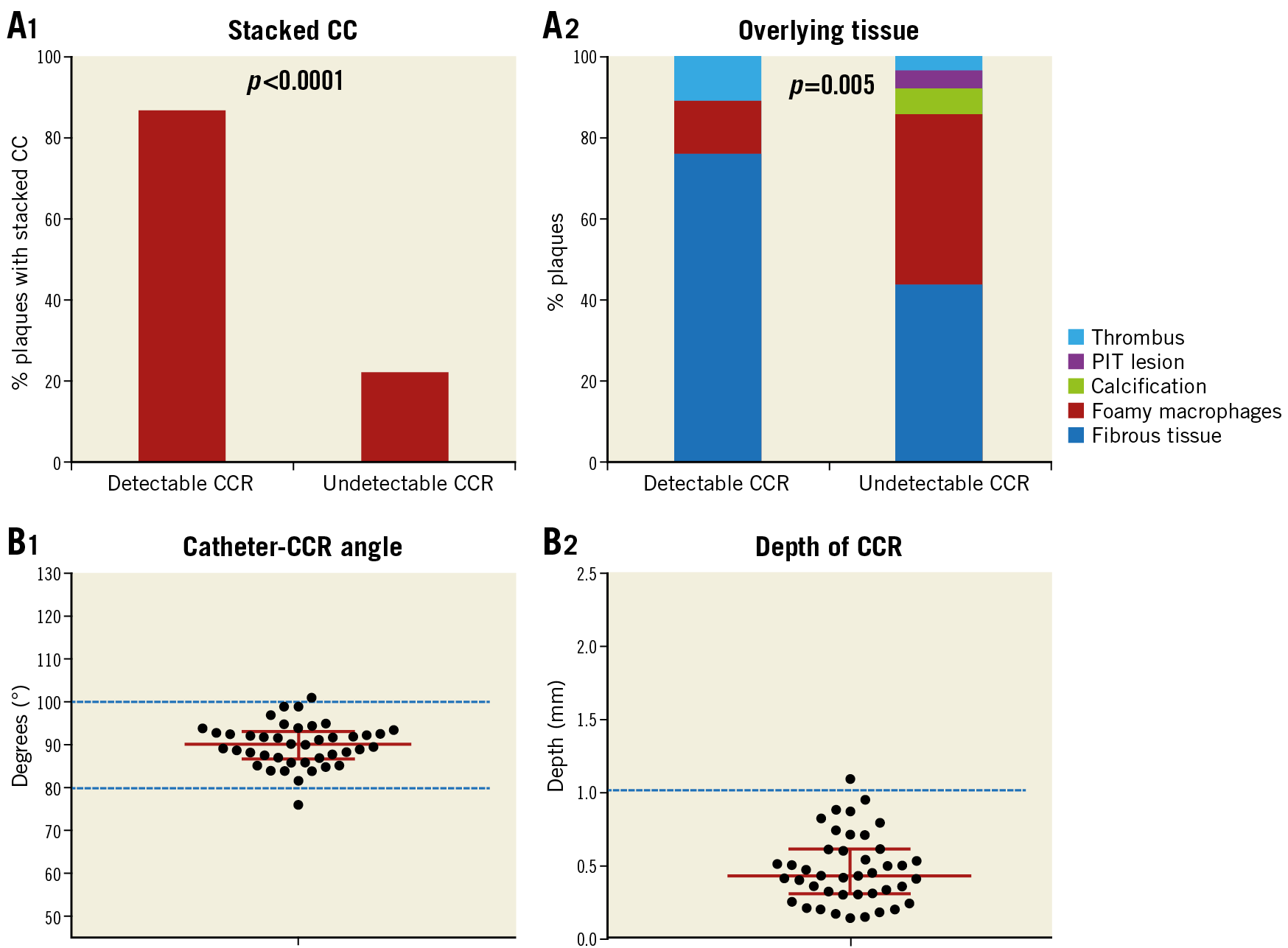
Figure 5. Histological characteristics between detectable and undetectable CCR and OFDI/OCT findings of CCR. A1) Percent stacked CC in detectable and undetectable CCR by OFDI/OCT. A2) Overlying tissue characteristics in detectable and undetectable CCR by OFDI/OCT. B1) >95.0% CCR were seen at a catheter-CCR angle of 90±10°. B2) Depth of CCR from surface with majority located within 1 mm. Detectable CCR (n=30) and undetectable CCR (n=87). CC: cholesterol clefts; CCR: cholesterol crystals; OCT: optical coherence tomography; OFDI: optical frequency domain imaging; PIT: pathological intimal thickening
OFDI/OCT MEASUREMENTS OF DETECTABLE CCR
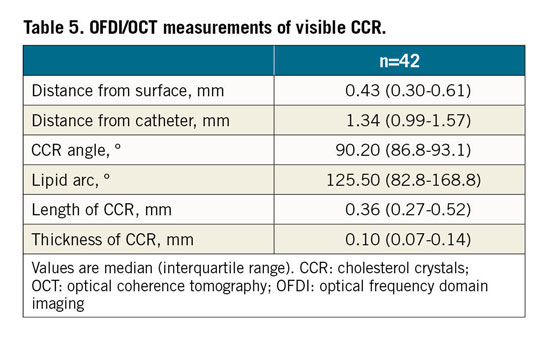
In total, 42 CCR were observed in 30 frames. The depth of detectable CCR by OFDI/OCT was 0.43 mm (median, interquartile range [IQR]: 0.30-0.61 mm), and >95% of the CCR were less than 1.0 mm in depth. Catheter-CCR angle was 90.2° (median, IQR: 86.8-93.1°) and >95.0% CCR were seen at a catheter-CCR angle of 90±10° (Table 5, Figure 5B).
MULTIVARIATE ANALYSIS FOR DETECTION OF CCR USING HISTOLOGICAL PARAMETERS
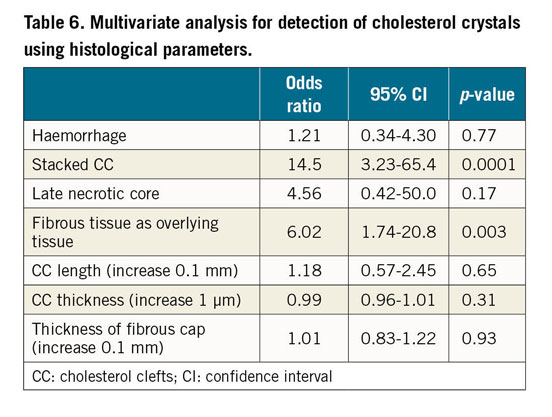
In univariate analysis, haemorrhage, stacked CC, late necrotic core, overlying fibrous tissue presence, CC length and thickness of fibrous cap showed significant differences between detectable and undetectable CCR by OFDI/OCT. Using these variables, multivariate analysis demonstrated that the presence of stacked CC and overlying fibrous tissue (Table 6) was highly predictive of OFDI/OCT identification of CCR.
Discussion
MAIN FINDINGS
This study is the first to examine the accuracy of OFDI/OCT for identification of the presence of CCR. In this study the primary findings were: 1) detection of CCR was low by OFDI/OCT; 2) advanced types of plaque (late FA, TCFA, and rupture) showed the presence of CCR by OFDI/OCT, whereas it was extremely rare within early FA; 3) a significant predictor for the detection of CCR by OFDI/OCT was the presence of overlying fibrous tissue and stacked CCR; 4) detectable CCR by OFDI/OCT were located within 1 mm from the luminal surface; and 5) longitudinal lines of the OFDI/OCT catheter sensor had to be close to 90° to the direction of CCR in order to visualise them.
Taken together, detectable CCR on OFDI/OCT mostly indicates the presence of stacked CC by histology and the presence of more advanced atherosclerotic lesions such as late necrotic core, presence of haemorrhage and large CC. Also, the detection of CCR was limited to advanced plaques; the presence of foamy macrophages or calcification above the CCR hindered the recognition of CCR by OFDI/OCT. Also, only CCR fulfilling specific optical conditions are detectable, i.e., angle and depth of OFDI/OCT are extremely important for the ability to recognise CCRs.
FREQUENCY OF OFDI- AND OCT-DERIVED CCR
As atherosclerosis progresses, plaque morphology changes based on stages of lesions. FA is the first clearly distinguishable plaque composed of lipid-rich necrotic core which consists of CC and cellular debris1. FA has been further subdivided histologically into early and late stages1. The former show presence of focal areas of necrotic core consisting of small CC and loss of proteoglycans and collagen in areas of macrophage infiltration, whereas the late FA show large areas of necrotic core composed of increasing numbers and larger size of CC, with complete lack of the extracellular matrix and collections of acellular debris1. TCFA and rupture also show presence of advanced necrotic core such as late FA; however, not all these lesions show OFDI/OCT-detectable CCR. Several clinical OCT/OFDI studies in living patients have detected the presence of CCR: these have varied from 17 to 24% in non-culprit sites6,7. CCR have been shown to be more frequent in acute coronary syndrome (ACS) patients and are recognised in 29-40% of culprit lesions requiring percutaneous coronary intervention for ACS or stable angina pectoris4,5,8. However, in clinical practice all types of lesion characteristics such as lipid pools, necrotic core, haemorrhage, and rupture are encountered. OFDI/OCT cannot distinguish between lipid pool and necrotic core. Therefore, the presence of CCR by OFDI/OCT should be helpful to identify these CCR lesions as necrotic cores, which would help to distinguish them from PIT lesions which do not contain CCR.
Clinical presentations may also affect plaque components. A meta-analysis reported by Iannaccone et al showed that the prevalence of TCFA and rupture was higher in order of ST-elevation myocardial infarction (STEMI) >non-STEMI >unstable angina >stable angina9. Therefore, the manner of clinical presentation might also affect the prevalence of CC detection by OFDI/OCT. In our study, only 25.6% of sections with areas of necrotic core showed presence of CCR by OFDI/OCT. On the other hand, plaque rupture was present in five cases, three of which had CCR presence, thus indicating that the presence of CCR on OFDI/OCT may predict more advanced plaques that are likely to rupture. However, the rate of detection for CCR in TCFA was low; however, this may be due to the overlying tissue being rich in foamy macrophages, which hinders the identification of CCR by OFDI/OCT.
FACTORS TO DETECT OFDI/OCT-DERIVED CCR
Prior to our study, the significance of the presence of detectable CCR as detected by OFDI/OCT was unclear, although many OFDI and OCT studies included CCR as one of the atherosclerotic parameters assessed. This study showed factors that determine the visibility of CCR, i.e., presence of stacked CC and fibrous tissue overlying the CCR. Abela et al reported individual CCR measured by scanning electron microscopy and reported that the diameter of crystals varied from 1-3 µm at the tips and from 50 to 150 µm in length, and were up to 20 µm wide at the base10. This study also measured the longest length of CCR: the median was 250 µm (IQR 150 to 340 µm). However, because the resolution of OFDI/OCT is 10 to 20 µm, it is not surprising that most CCR cannot be identified as the width is <5 µm. We identified that stacked CC was the best predictor of the presence of CCR, probably due to their collective thickness exceeding 15 µm. Also, the type of tissue overlying CCR is important, with fibrous tissue allowing greater penetration while calcium and foamy macrophages hamper this penetration. This leads to the attenuation of the signals which results in the inability of OFDI/OCT to identify CCR. Late necrotic core typically shows increase in the numbers of single CCR in histological sections, and the presence of haemorrhage also contributes to the increase of CCR, as reported previously11, which may allow OFDI/OCT to identify CCR.
CLINICAL IMPLICATION OF CCR
Intracoronary imaging devices do not possess the capability to distinguish lipid pools clearly in PIT lesions or the necrotic core in early FA from that in late FA. The presence of CCR indicates not simply necrotic core but more advanced necrotic core. The presence of OFDI/OCT-identified CCR from our study clearly shows that it reflects the presence of late FA. Our study shows that almost all detectable CCR are located in late necrotic core. Also, CCR correlated with the presence of plaque haemorrhage. Therefore, almost all lesions that show the presence of CCR by OFDI/OCT are advanced necrotic cores, and often show the presence of haemorrhage. In order to improve risk stratification and to guide therapy to prevent untoward cardiovascular events, the information from CCR may help us to understand the composition of advanced necrotic cores within plaques. Their presence by OFDI/OCT signifies an advanced lesion.
Limitations
First, all autopsy hearts were interrogated by intracoronary imaging devices in a blood-free environment, which is different from conditions in the catheterisation laboratory. However, all hearts were imaged during pressure perfusion with saline flush before formalin fixation; therefore, we believe that our findings remain valid and relevant to in vivo detection. Second, the study included non-cardiac and non-coronary death cases and a large majority of lesions were from stable sudden coronary death. Therefore, the incidence for the detection of CCR may be lower than if high-risk patients presenting with ACS had been included in the study.
Conclusions
OFDI/OCT is able to detect CCR within advanced atheromatous plaques although the sensitivity of OFDI/OCT for their detection was relatively low with higher specificity. The sensitivity was markedly improved by the presence of stacked CC with overlying tissue which attenuates the light signal. In addition to these two factors, optical conditions such as shallow depth and specific angle of CCR are important for the detection of CCR. The presence of CCR by OFDI/OCT suggests that the lesions are advanced.
|
Impact on daily practice By histologic assessment at autopsy, detection for cholesterol crystals (CCR) by OFDI/OCT indicates stacked cholesterol clefts. The presence of CCR probably indicates advanced necrotic core (i.e., late necrotic core and plaque haemorrhage). This information from OFDI/OCT-detected CCR may help us to identify complicated atherosclerotic plaque in coronary arteries and improve risk stratification. |
Funding
This study was funded by CVPath Institute, Inc., Gaithersburg, MD, USA.
Conflict of interest statement
R. Virmani has received institutional research support from Abbott Vascular, Boston Scientific and Terumo Corporation, honoraria from Abbott Vascular, Boston Scientific and Terumo Corporation, and is a consultant to Abbott Vascular, Boston Scientific and Terumo Corporation. A.V. Finn has received institutional research support from Abbott Vascular, Boston Scientific and Terumo Corporation, honoraria from Abbott Vascular, Boston Scientific and Terumo Corporation, and is a consultant to Abbott Vascular and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.