Vascular calcification is one of the important components of arteriosclerosis. Calcification is frequently observed in coronary arteries with advanced arteriosclerosis. Intravascular optical coherence tomography (OCT) can clearly visualise coronary calcification in vivo. OCT images of coronary calcification are characterised by well-delineated, signal-poor regions with sharp borders. Coronary calcifications are morphologically classified as calcium sheet or nodule. Nodular calcifications protruding into the coronary lumen (called calcified nodules) are found in the culprit lesion of acute coronary syndrome1. Furthermore, such protruding nodular calcifications are also identified in non-culprit lesions. A limited number of studies have evaluated the pathophysiology of calcified nodules.
Present study
In the current issue of EuroIntervention, Prati et al2 report the clinical outcome of calcified nodules identified by OCT in non-culprit coronary lesions.
The authors demonstrated that the patients who had calcified nodules with disruption of the superficial intimal fibrous layer were at increased risk of subsequent cardiac events2. This result suggests that OCT is helpful in stratifying patient event risk through detection of calcified nodules.
Calcified nodules
Pathology studies have reported calcified nodules to be one of the unstable plaque features observed in coronary thrombotic lesions responsible for acute coronary syndrome. A calcified nodule is characterised by small rounded calcified fragments separated by fibrin with luminal protrusion, fibrous cap disruption, and overlying thrombi3. Compared with plaque rupture and erosion, calcified nodules were least frequently found in cases of sudden coronary death. So far, the development mechanism of calcified nodules is not precisely known, because pathological studies are limited to assessments after coronary thrombosis.
OCT is the most promising intravascular high-resolution imaging for identifying calcified nodules in the clinical setting (Figure 1). An OCT-derived calcified nodule is defined by fibrous cap disruption detected over a calcified plaque characterised by protruding calcification, superficial calcium, and the presence of substantive calcium proximal and/or distal to the lesion4. The OCT definition certainly includes important pathological characteristics of a calcified nodule. An OCT-derived calcified nodule is often found in the culprit lesions of non-ST-segment elevation acute coronary syndrome in elderly patients with hypertension and chronic renal disease4. As OCT imaging can be performed repeatedly at different times in a living patient, this method is useful for understanding the origin and outcomes of calcified nodules.
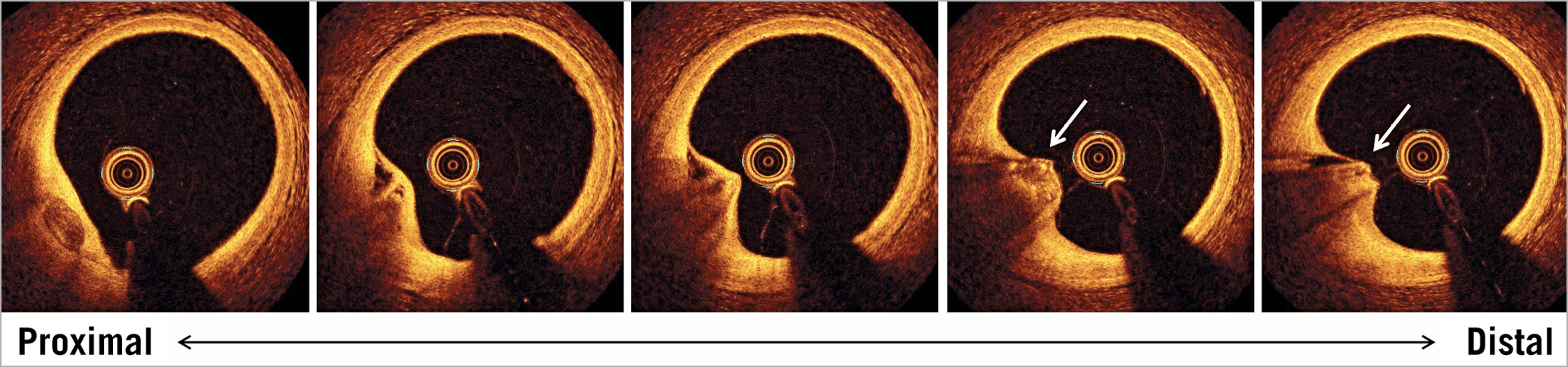
Figure 1. An example of an OCT-derived calcified nodule. OCT images were obtained in the mid RCA of a 76-year-old man with stable coronary artery disease. A nodular calcification protrudes into the lumen. Fibrous cap disruption is identified by the discontinuity of a thin bright layer overlying the nodular calcification (arrows). There is no significant lumen narrowing. OCT: optical coherence tomography; RCA: right coronary artery
OCT-derived calcified nodules are also found in non-culprit coronary lesions. The study by Prati et al2 demonstrated a possible link between OCT-derived calcified nodules and subsequent lesion-specific myocardial infarction. OCT-derived calcified nodules may have a progressive nature. One possible cause of calcified nodule progression may be vascular motion such as contraction, dilation and hinge motion. Intense vascular motion may break the calcium sheet and induce small nodular calcification. Further, the vascular motion may cause small nodular calcifications to protrude into the lumen and ultimately disrupt the fibrous cap. Calcification is thrombogenic and thus the nodular calcifications can lead to intraluminal thrombosis. The risk of thrombosis might be higher in calcified nodules with fibrous cap disruption than in those with intact fibrous cap. Comparison of OCT images in calcified nodules between baseline and the time of a subsequent thrombotic event will help to understand the histopathological changes that have occurred in the lesion during the follow-up period.
In the past, vascular calcification has been recognised as a stable lesion in the terminal stage of arteriosclerosis. However, the calcification is not always benign. An OCT-derived calcified nodule with fibrous cap disruption in non-culprit lesions may be a precursor of coronary thrombosis.
The study by Prati et al2 demonstrated an increased risk of cardiac death in patients with OCT-derived calcified nodules in non-culprit lesions. The cardiac death in the study2 appeared to be associated with coronary events arising from lesions other than OCT-derived calcified nodules. The presence of OCT-derived calcified nodules may represent advanced arteriosclerosis throughout the coronary arteries. Previous OCT studies showed that large lipid core, thin fibrous cap, macrophage accumulation, and small lumen area were predictors for future cardiovascular events at a patient level5. The combination of an OCT-derived calcified nodule with these traditional predictors is expected to improve the accuracy of OCT for identifying patients at high risk for future cardiovascular events.
Currently available therapies may not be effective in reducing the risk of future coronary events associated with OCT-derived calcified nodules. Lipid-lowering therapy is unlikely to be effective, as lipids are not involved in the progression of calcified nodules. Also, stenting may not be a feasible solution because nodular calcifications can protrude through the stent struts and lead to early and late in-stent failures6. Further research is needed to find effective treatments to improve clinical outcomes in patients with OCT-derived calcified nodules.
Final remarks
OCT-derived calcified nodules in non-culprit lesions appear to be a predictor of clinical outcomes in patients with coronary artery disease. The challenge is how to use this information in daily clinical practice.
Conflict of interest statement
T. Kubo has received lecture fees from Abbott Vascular Japan. T. Akasaka has received lecture fees and research funds from Abbott Vascular Japan.
Supplementary data
To read the full content of this article, please download the PDF.