
Optical coherence tomography (OCT) was introduced into cardiovascular imaging in 19971, emerging as a spin-off from an original scientific invention within the field of ophthalmology to enable imaging of retinal microstructures including arterioles and to facilitate detection of early degenerative changes of retinopathies. Consequently, OCT was praised as being a ground-breaking imaging technology which provides a far superior resolution as compared to the already established ultrasound-based imaging system (intravascular ultrasound [IVUS]). As such, it provides high-resolution tomographic images (10-20 µm), which enable recognition of atherosclerotic plaque patterns2,3, and can be used to identify high-risk lesions including their thrombotic complications. More specifically, it allows measurement of fibrous cap thickness and is used to identify high-risk coronary plaques that are prone to disruption. However, limited penetration of light, especially in diseased vessel wall segments, has been the Achilles’ heel of OCT imaging in coronary arteries. Distribution of light when passed through a tissue is predicated on its intrinsic optical properties. Lipid-rich tissue displays a high level of attenuation and has a low penetration depth, making it difficult to identify underlying plaque morphology correctly. This fact makes OCT penetration depth in arteries dependent on tissue type and limits correct classification of lesions where lipid content is high.
Against this background, in this issue of EuroIntervention, Poulsen et al report the findings of a preclinical study comparing plaque morphology in the coronary arteries of five transgenic hypercholesterolaemic mini-pigs using OCT with co-registered histology4.
A total of five shear-modifying stents were implanted into the left anterior descending or left circumflex artery to induce endothelial denudation and formation of atherosclerotic lesions. Angiography and intravascular in vivo OCT follow-up were performed after 34 weeks in four animals, while one animal received follow-up investigations prior to death at 19 weeks of follow-up. Plaque morphology by OCT was assessed in 201 OCT frames according to the current consensus standards and compared to histology using plaque classification established by Virmani et al5. The authors discovered a high sensitivity but modest specificity (92.9% and 74.6%, respectively) for identifying fibrous cap atheroma (FCA) by OCT as compared to histology. The reduced specificity for OCT was explained by misclassification of plaques with histologically defined pathological intimal thickening (PIT) as FCA (46.1% of the frames with histological PIT were misclassified as FCA). Misclassified lesions had a higher plaque burden by histopathology as compared to appropriately classified lesions (median 32.0% versus 13.4%; p<0.0001). Misclassification of PIT lesions as FCA by OCT occurred when the plaque burden in a particular frame exceeded approximately 20%. The authors discuss how the specificity of in vivo OCT analysis to identify fibroatheroma was hampered by foam cell accumulation close to the luminal surface and reduced backscatter of light due to its lipid content. Furthermore, there was remarkably reduced sensitivity of OCT to detect thin- vs. thick-cap fibroatheroma relative to the gold standard histology owing to incorrect measurement of fibrous cap thickness by OCT when using traditional cut-off values derived from seminal autopsy studies (sensitivity and specificity 28.6% and 96.4% for thin-cap fibroatheroma, 66.7% and 73.9% for thick-cap fibroatheroma, respectively)5. The authors conclude by underlining the strength of OCT imaging, with its high sensitivity to detect FCA, counterbalanced by its relative weakness in specificity secondary to limited light penetration depth in lesions with significant plaque burden.
First and foremost, the authors must be congratulated for performing tedious co-registration studies of OCT against its gold standard histopathology in diseased animals, which has been an unmet need to date and helps to identify the clinically relevant shortcomings of OCT imaging in detecting high-risk atherosclerotic lesions. Such endeavours are of importance because there has been significant downtime in progressing advanced intravascular imaging methodologies towards detection of patients at risk of future cardiovascular events. Studies such as the current one help to identify translational gaps and common misconceptions applicable to most cardiovascular imaging modalities and subsequently facilitate technological iteration with fine-tuning of highly relevant diagnostic tools.
However, some important limitations of the current study must be considered in order to aid interpretation of the most salient findings in relation to the use of OCT in daily clinical practice.
Firstly, the current study was conducted in a genetically engineered large animal model transgenic for proprotein convertase subtilisin/kexin type 9 (PCSK9), subsequently enabling in vivo imaging of atherosclerotic lesions. However, it must be stressed that, despite all the efforts put into the breeding and high-cholesterol feeding of these animals, human-like atherosclerotic lesions including aspects of plaque vulnerability and calcification cannot be replicated in these models. Lesions in these animals are usually rich in foamy macrophages and extracellular lipid pool, while true necrotic core with extravasation of red blood cells, accumulation of cholesterol clefts and additional cellular debris arising from defective efferocytosis6 are rarely observed (Figure 1). Furthermore, active thinning of the fibrous cap by infiltration of monocytes and neutrophils with breakdown of extracellular matrix and weakening of collagen are key phenomena related to plaque vulnerability in man and unknown to occur in a similar fashion in animal models of accelerated disease conditions. Consequently, the results of the current study are to be interpreted with caution since it remains unclear whether the observed limitation of OCT to differentiate FCA is secondary to excessive foamy macrophage formation and lipid pool within diseased segments of porcine coronary arteries, which are known to cause significant light attenuation during OCT imaging7. Hence, the putative mechanism of the lack of specificity to differentiate FCA put forward by the authors seems reasonable since macrophages represent a major limitation for the appropriate delineation of underlying plaque morphology. Along these lines, atherosclerotic plaque involving substantial plaque burden by histopathology is unlikely to be mirrored appropriately by OCT, since scattering of light during its passage through stenotic vascular segments is quite significant, a phenomenon already encountered and reported in human autopsy studies (Figure 1)8,9.
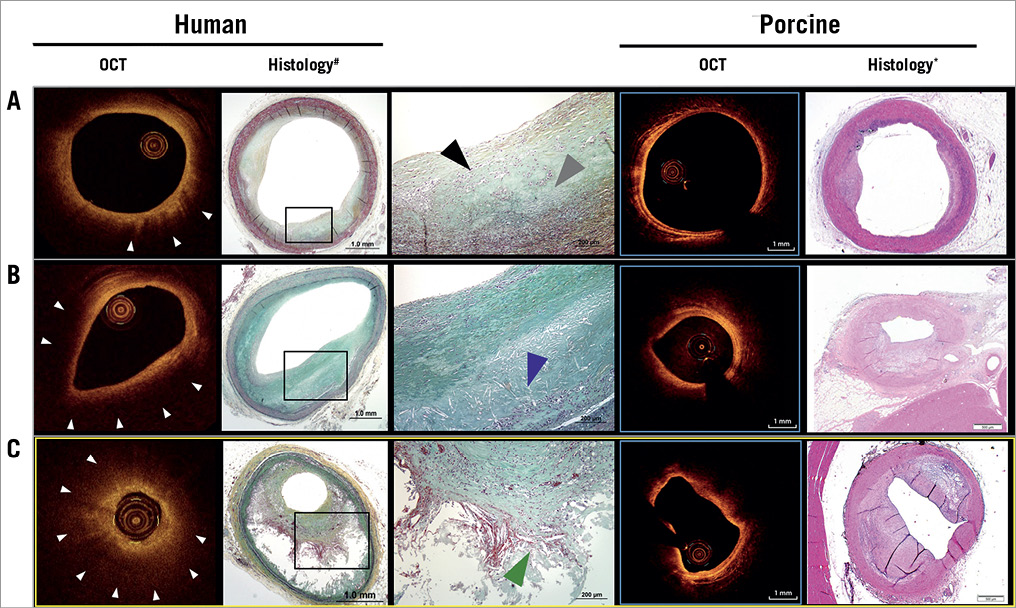
Figure 1. Comparison of co-registration studies performed in human autopsy specimens with matched OCT frames and histological cross-sections of pathological intimal thickening (PIT, A), early fibroatheroma (B) and late stage fibroatheroma with thick fibrous cap (C, yellow frame) and corresponding sections derived from Poulsen et al4. While early stages of atherosclerosis (A & B) can be reliably reproduced in diseased animal models, late stages (C) of atherosclerosis cannot be appropriately mirrored in contemporary diseased animal models. Note, there is intra-plaque haemorrhage, cholesterol clefts and cellular debris resulting from defective efferocytosis in late-stage fibroatheroma, which is not observed in preclinical animal models. Also, the presence of significant necrotic core (C) and/or macrophages (B) causes unclear delineation of underlying plaque morphology. White arrowheads point to significant attenuation of the OCT signal owing to the presence of macrophages; the black arrowhead points to the presence of macrophages, while the grey arrowhead denotes the presence of lipid pool; the blue arrowhead points to early necrotic core formation; the green arrowhead denotes the presence of intra-plaque haemorrhage within the necrotic core. *H&E stain. #Movat’s pentachrome stain. Reprinted with permission from4. Modified and reprinted with permission from8.
Validation of OCT by histology has, however, been achieved with a rather high degree of specificity in native plaques. One such important study by Yabushita et al10 correlated OCT images with histological data obtained from 357 diseased arterial segments from 90 randomly selected cadavers. The duration between OCT imaging and time of death was less than 72 hours. They assessed 357 OCT images with their co-registered histological cross-sections. Agreement between the pre-set OCT image criteria (fibrous plaques, fibrocalcific plaques and lipid-rich plaques) and histopathological diagnosis for corresponding sections was found to be high (Cohen’s kappa coefficient of concordance, 𝜅=0.83 to 0.84). Predominantly fibrous plaques, which had small amounts of lipid, were wrongly classified as lipid-rich plaques. These visually identified lipid-rich plaques in OCT cross-sections were later confirmed as fibrous plaque by histopathology, and resulted in low sensitivity of the OCT criteria for the diagnosis of fibrous plaques. More recent publications have reported substantial limitations of OCT to detect plaque morphology appropriately. In a study by Rieber et al11, moderate agreement between OCT and histology (𝜅=0.67) was reported. This was confirmed in another study by Manfrini et al12 who demonstrated that OCT could not differentiate selective tissue components such as lipid and calcification. It must be mentioned that detailed assessment of atherosclerotic tissue components could not be performed in the current study owing to a lack of advanced atherosclerotic lesions in the diseased porcine animal model.
Several limitations on the study of human cadavers should be acknowledged. In autopsy studies, absence of heartbeat during OCT image acquisition, high-pressure flushing and residual blood in the vessel lumen could have resulted in incorrect depiction of atherosclerotic plaque components. Hence, the ideal model to perform sophisticated co-registration studies to validate intravascular imaging against histopathology remains to be determined.
Secondly, intravascular imaging using OCT was performed in an artificial environment including flow-divider stent implantation to foster formation of atherosclerotic lesions. Imaging settings may be different in this animal model, where high-grade stenosis upstream and downstream from imaged vascular segments may have created abnormalities in flow dynamics relevant to plaque formation and imaging.
Thirdly, OCT analysis and adjudication of lesions were performed blinded to histology, causing concerns as to whether this factor alongside the different sampling rate between histology (60 µm) and OCT (200 µm) may have caused misalignment of co-registered histology sections and OCT frames. Nevertheless, there is also a strong argument for performing OCT assessment blinded to histopathology, since knowledge of the underlying disease pattern by histopathology may probably have influenced adjudication status.
In summary, the authors have certainly contributed to improving our understanding of common misconceptions between intravascular OCT imaging and histopathology, which is of high clinical relevance given the transition into an era of individualised medicine based on precision imaging. Relevant progress to achieve this milestone can only be accomplished when major ambiguities during imaging of high-risk atherosclerotic lesions have been resolved.
Conflict of interest statement
M. Joner reports being a consultant for and receiving speaker fees from Biotronik and OrbusNeich. The other authors have no conflicts of interest to declare.

