
For the development of clinical atherosclerotic disease, nothing is more dangerous than living a long life. The longer you survive other threats, the more likely you are to suffer the consequences of a life’s exposure to atherosclerosis risk factors. The upsurge in lifespan during the last century, achieved by improvements in socio-economic conditions and health care, has been the central cause of the rise in cardiovascular disease across the world1. Because atherosclerosis remains harmless for a long time, there is no need to avoid its development. Rather, the goal should be to retard it sufficiently to make death from atherosclerosis an acceptable alternative to other causes. But how can we know whether our vessels are on the right track? And how can we know early enough to be able to steer them back on track if they are not? To achieve this we need to understand and be able to monitor the development of dangerous lesions.
Plaque necrosis is arguably the most detrimental feature of atherosclerosis. Without it, atherosclerosis would rarely cause problems. It is not only the presence of a necrotic core which is necessary for the process of plaque rupture to occur. In a helicopter view, the development and rupture of thin-cap fibroatheromas (TCFAs) may simply be understood as growth of a necrotic core until it reaches the surface. The inflammation and proteolytic degradation that finally thins and weakens the fibrous cap to breaking point has been the object of much interest, but it is the location and not the nature of these mechanisms that stands out. Similar processes may occur generally where intimal tissue is converted into necrotic core2, and their presence in the thinning fibrous cap may simply signify that this piece of intima is next in line.
There has been some interesting work on the molecular causes of plaque necrosis in recent years, but the dynamics of core development in humans and the reasons for its variability among different vascular beds and individuals are largely unresolved. Long-term monitoring of vessels throughout the natural history of TCFA development in humans has not been possible; instead, we are left to try to make sense of separate snapshots of coronary arteries from autopsies. From this, we know that the proximal parts of coronary arteries develop lesions with lipid pools and necrosis in early adulthood3, and the same segments harbour the majority of TCFAs, plaque ruptures and symptomatic thrombi when they begin to appear later in life4,5. Does this mean that the lesions that first develop necrosis are the same as those that later rupture as TCFAs? The new study by Saybolt et al, using an accelerated atherosclerosis model in diabetic and hypercholesterolaemic pigs, indicates that this is a likely scenario6.
In their technically taxing experiment, they found that coronary arteries with TCFAs had significantly increased levels of lipid deposition three months previously as measured by near-infrared spectroscopy (NIRS) and quantified by lipid core burden index (LCBI). Based on prior validation of NIRS in the same pigs7, and supported by coinciding changes in echo-attenuated plaque area by IVUS, this probably reflected differences in the rate of necrotic core formation.
The main strength of this study is that it interrogated plaque development in the same coronary arteries several times during the natural history of atherosclerosis. Also, not only did the pigs develop many TCFAs (although using a relaxed criterion of <100 µm fibrous cap thickness), but they also presented with acute thrombotic events. Two examples are presented in the paper. While both fall short of revealing the cause of thrombosis, one of them shows loss of material within a lesion, potentially caused by extrusion of necrotic core material through a rupture in a neighbouring location. It would be interesting to analyse in more depth whether the thrombi seen in the diabetic and hypercholesterolaemic pig model share mechanisms with human thrombus formation.
Importantly, although no formal analysis of the ability of LCBI to predict the development of TCFA was performed, the level of LCBI measurements at six months showed considerable overlap between TCFA developers and TCFA non-developers, and hence the LCBI measure provided only partially predictive information. This could reflect limitations in what is measured (lipid accumulation), the technique to measure it (NIRS), or the inherent unpredictability of lesion development. Even in experiments, such as the one by Saybolt et al, where care is taken to keep life conditions stable and the number of lifestyle choices is clearly minimal, there is considerable variation in the course of coronary lesion development. It is fundamentally unknown to what extent the development of TCFAs could be predicted provided we had the right information, or whether the course of lesion development has elements of randomness. Previous studies have shown that anatomic differences leading to differences in blood flow patterns can influence the development of TCFAs in the coronary arteries of pigs7,8, and much of this anatomic variability does not appear to be genetically determined, but could be random9. Imaging of the coronary anatomy might solve this, but also within plaques there is the possibility that small random variations may be amplified during plaque development, making it inherently impossible to predict necrosis and TCFA development with certainty deep into the future (Figure 1).
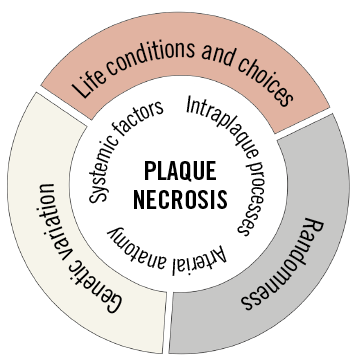
Figure 1. Causes and mechanisms determining the rate of necrotic core growth. Lifestyle and genetic disposition acting through conventional systemic risk factors and intraplaque processes are clearly important, but the coronary anatomy also appears to play a decisive role. The level of randomness in the process is not known.
NIRS has recently been studied for its ability to risk stratify patients after coronary interventions for ACS and stable angina, and, while it is clear that NIRS provides prognostic information on the risk of future events compared to having no prior information10, it remains to be clarified whether NIRS provides information additional to that given by plaque burden. How should the study by Saybolt et al be compared with these potential human applications of NIRS? At first glance, predicting three months into the future may appear an easier task than doing the same thing over years in humans. However, it is probably a considerably harder challenge. Since the bulk of lesion development in the pigs occurred over only about six months, a more reasonable description of the task was to predict the development of TCFAs halfway through their genesis and at a stage where the lesions (and the animals) were considerably smaller than at the endpoint. In this respect, Saybolt et al do something that has never been attempted in humans: analysing vessels in the young and trying to predict how fast the development towards dangerous lesions is going. The balance of changes in life expectancy and the rate of dangerous atherosclerotic lesion formation will determine the future burden of atherosclerosis. Experiments such as the one by Saybolt et al can teach us to monitor where we are heading.
Funding
The CNIC is supported by the Spanish Ministry of Economy and Competitiveness (MINECO) and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505).
Conflict of interest statement
The author has no conflicts of interest to declare.

