Abstract
Aims: The presence of thin-cap fibroatheromas (TCFA) is associated with high risk of acute coronary syndrome, hence their early detection may identify high-risk patients. In the present study we investigated the ability of a combined imaging catheter with near-infrared spectroscopy (NIRS) plus intravascular ultrasound (IVUS) to detect TCFA in patients with stable coronary artery disease.
Methods and results: Optical coherence tomography (OCT) and combined NIRS-IVUS assessment were performed on identical coronary segments. IVUS analysis provided per-segment minimal cross-sectional area (CSA), plaque length (PL), plaque burden (PB), plaque volume (PV), and remodelling index (RI). OCT was used as the gold-standard reference to define TCFA (fibrous cap thickness <65 μm). Plaque lipid content was estimated by NIRS (lipid core burden index [LCBI]). OCT-defined TCFA was present in 18 of 76 segments. IVUS revealed that OCT-defined TCFA were positively remodelled lesions with greater PB and PV, smaller CSA, and longer PL, while NIRS revealed greater LCBI per 2 mm segment (LCBI2mm) (all p<0.001). Greatest accuracy for OCT-defined TCFA detection was achieved using LCBI2mm >315 with RI >1.046 as a combined criterion value.
Conclusions: OCT-defined TCFA are characterised by positive vessel remodelling, high plaque burden and greater lipid core burden as assessed by dual NIRS-IVUS imaging.
Introduction
Atherosclerotic coronary artery plaque rupture leads to intravascular thrombus formation, which may culminate in an acute coronary event with myocardial ischaemia. Rupture-prone atherosclerotic plaques are known as “vulnerable plaques”. They are characterised by outward vessel expansion (positive remodelling), a high burden of intra-plaque lipid, macrophage infiltration and a thin fibrous cap (<65 µm)1-3. Histopathologically, these plaques are referred to as “thin-cap fibroatheromas” (TCFA). The presence of TCFA is associated with increased risk of acute coronary syndrome4, hence their early detection may help to stratify high-risk patients.
Optical coherence tomography (OCT) is the principal imaging tool used to detect TCFA. The high imaging resolution of OCT (10-20 µm) permits the measurement of fibrous cap thickness and also the classification of plaques as being fibrous, calcified or lipid-rich5. However, OCT is limited by its low depth penetration into the vessel wall (2 mm), which hampers accurate assessment of necrotic core depth and vessel remodelling6-8. Moreover, OCT imaging requires the evacuation or replacement of blood from the vessel, and it is poorly suited to assessing aorto-ostial coronary lesions. As an alternative modality, greyscale intravascular ultrasound (IVUS) imaging does not require the evacuation of blood and has superior depth penetration with a unique ability to measure plaque burden and vessel remodelling9. However, due to substantially inferior resolution (100-150 µm), IVUS is unable to measure cap thickness to the required accuracy to diagnose TCFA directly10, and it does not provide distinction between lipid-rich and non-lipid-rich plaques11.
The recent combination of near-infrared spectroscopy (NIRS) with greyscale IVUS in a single imaging catheter allows simultaneous assessment of plaque composition both in terms of its chemical (by NIRS) and morphologic (by IVUS) characteristics. Specifically, NIRS provides the ability to quantify plaque lipid content12,13. The validity of this dual modality catheter was provided by prior studies in the setting of acute coronary syndrome, which documented that, while NIRS detects plaque composition14, greyscale IVUS describes morphology15. A previous autopsy study demonstrated that NIRS is able to detect features associated with plaque vulnerability in humans14. In the present study, we investigated the ability of combined NIRS-IVUS imaging to detect OCT-defined TCFA in patients with stable coronary disease.
Methods
STUDY POPULATION
The current study employed intravascular OCT and combined NIRS-IVUS assessment of identical segments of the same patients’ coronary arteries/plaques. All imaging was performed during the same procedure. The study group was selected from patients referred for cardiac catheterisation due to chronic stable coronary disease symptoms. Patients with renal failure (creatinine >1.5 mg/dl), haemodynamic compromise, contrast allergy, and aorto-ostial coronary artery lesions were excluded from the study. The segment to scan was selected at the operator’s discretion after the diagnostic angiogram. One segment of coronary artery was defined as the region between two significant side branches. Analysed segments were both target and non-target regions in the imaged artery by OCT and NIRS-IVUS. None of the patients developed any complications due to OCT and combined NIRS-IVUS imaging, which were performed using bivalirudin anticoagulation (activated clotting time >300 sec) and following intracoronary nitroglycerine (100-200 µm) administration.
NIRS, IVUS and OCT images were analysed off-line together with angiographic quantitative coronary analysis (QCA). Coronary segments with incomplete and/or poor quality NIRS, IVUS or OCT scans were removed from the investigation (16 coronary segments), hence the ultimate analysis included 76 coronary segments assessed in 60 patients.
All patients were drawn from the Institutional Review Board (IRB) approved Clinical Interventional and Multimodality Imaging Database of The Mount Sinai Medical Center in New York, USA, and in accordance with the Code of Federal Regulations and the Declaration of Helsinki. All patients signed an informed consent before the procedure. All patients undergoing PCI are entered into this database within 24 hours of the index intervention. Details of the Clinical Interventional database have been published previously15,16.
QUANTITATIVE CORONARY ARTERIOGRAPHY MEASUREMENTS
QCA was performed off-line using a validated cardiovascular measurement system (CMS) (QAngioXA software 7.3; Medis, Leiden, The Netherlands) for calculation of minimum lumen diameter, reference lumen diameter and % diameter stenosis with complete coronary segment morphology characteristics.
OPTICAL COHERENCE TOMOGRAPHY IMAGE ACQUISITION
The commercially available intravascular imaging system OCT C7 Dragonfly™ (St. Jude Medical, St. Paul, MN, USA) was used for OCT image acquisition. OCT image analysis scrutinised serial cross-sectional images for plaque composition, cross-sectional area (CSA), and vessel diameter. Plaque composition was analysed according to previously validated criteria for OCT (Online Appendix)5,17. OCT-defined TCFA was defined as a lipid-rich plaque with fibrous cap thickness <65 µm.
COMBINED NIRS AND GREYSCALE IVUS IMAGE ACQUISITION AND ANALYSIS
Combined NIRS and greyscale IVUS image acquisition was performed using the commercially available TVC Imaging System™ with the 2.4 Fr Insight™ Catheter (Infraredx, Inc., Burlington, MA, USA). The NIRS analysis allows calculation of lipid core burden index (LCBI) (Online Appendix), and in the present study the maximal LCBI was estimated in 2 mm, 4 mm and 8 mm pullback compartments for every analysed lesion (LCBI2mm, LCBI4mm, LCBI8mm, Figure 1).
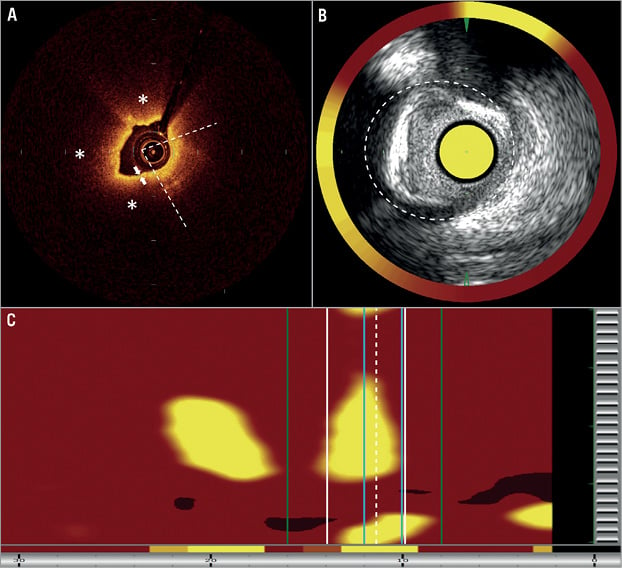
Figure 1. Representative case of OCT-defined TCFA for greyscale IVUS and near-infrared spectroscopy. A) Optical coherence tomography showed lipid-rich plaque (*) with lipid arc=330° (open angle between two white dashed lines) and fibrous cap thickness 50 µm (white arrows). B) Greyscale IVUS showed minimal cross-sectional area (CSA)=1.95 mm2, external elastic lamina (EEM) CSA=11.00 mm2 (white dashed line); estimated plaque burden was 82.27% and the vessel was positively remodelled (reference EEM CSA was 9.53 mm2). C) NIRS revealed the maximum LCBI2 mm=592 (2 mm segment between the blue lines), maximum LCBI4 mm=416 (4 mm segment between white lines) and maximum LCBI8 mm=279 (8 mm segment between green lines).
Quantitative greyscale IVUS measurements were performed using QIvus version 2.1 (Medis, Leiden, The Netherlands) to quantify lumen CSA, external elastic lamina (EEM) CSA, plaque and media CSA, plaque burden, lumen and vessel volume and EEM volume, and remodelling index (RI). Lesions with RI ≤0.95 were defined as negatively remodelled, while those with an RI ≥1.05 were defined as positively remodelled (Online Appendix).
CO-REGISTRATION OF NIRS, GREYSCALE IVUS AND OCT
During the combined NIRS-IVUS pullback, anatomical landmarks were imprinted on the chemogram and bookmarked on the IVUS images, i.e., fiducial points, minimal vessel CSA and side branches. Those landmarks allowed matching of OCT images to corresponding sections of NIRS map, IVUS images and the coronary angiogram. Such co-registration allowed simultaneous judgement of LCBI values, IVUS measurements and OCT analysis in the same lesion of the coronary vessel.
The statistical analysis was performed using MedCalc software version 12.2.1 (MedCalc, Ostend, Belgium) (Online Appendix).
Results
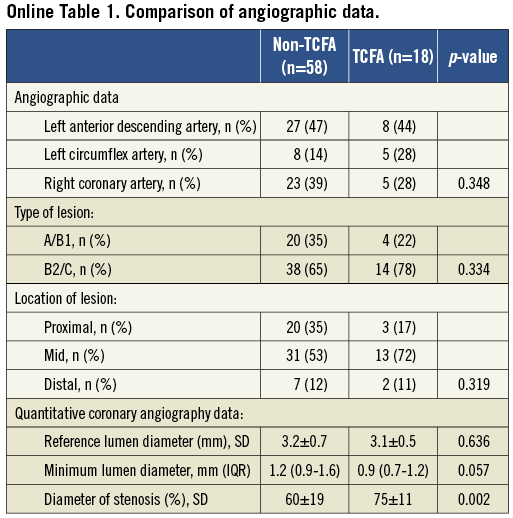
Sixty patients were enrolled into the study. Using OCT, we identified the presence of TCFA in 18 of 76 segments. There were no significant differences in clinical patient characteristics, pharmacological therapy and laboratory results between patients with and without TCFA (Table 1). Angiographic QCA analysis is summarised in Online Table 1. Coronary segments with OCT-defined TCFA were characterised by higher % diameter stenosis and tended to have smaller minimal lumen diameter assessed by QCA. There were no differences between the groups in terms of lesion location and type.
IVUS analysis revealed that lesions with OCT-defined TCFA were positively remodelled and had greater plaque burden, greater plaque volume, smaller minimal CSA, and longer length (Figure 2).
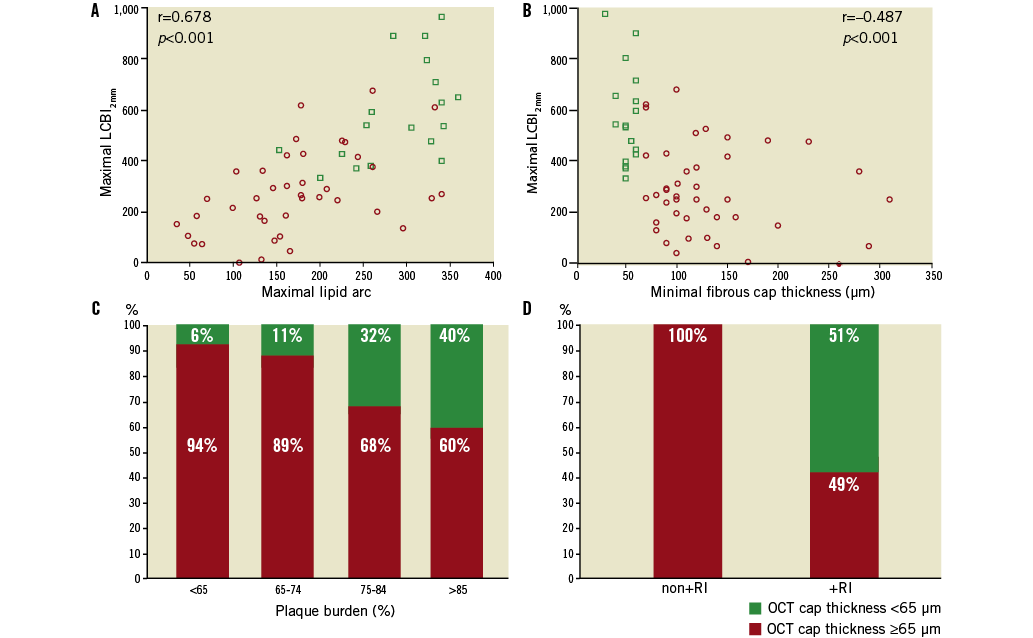
Figure 2. NIRS and IVUS parameters vs. OCT-defined plaque characteristics. A) Correlation between maximal LCBI2 mm vs. OCT-defined maximal lipid arc. B) Correlation between maximal LCBI2 mm vs. OCT minimal cap thickness. C) Prevalence of OCT-defined TCFA vs. IVUS-determined plaque burden. D) Prevalence of OCT-defined TCFA vs. IVUS remodelling index (+RI: positive vessel remodelling; non+RI: not positively remodelled).
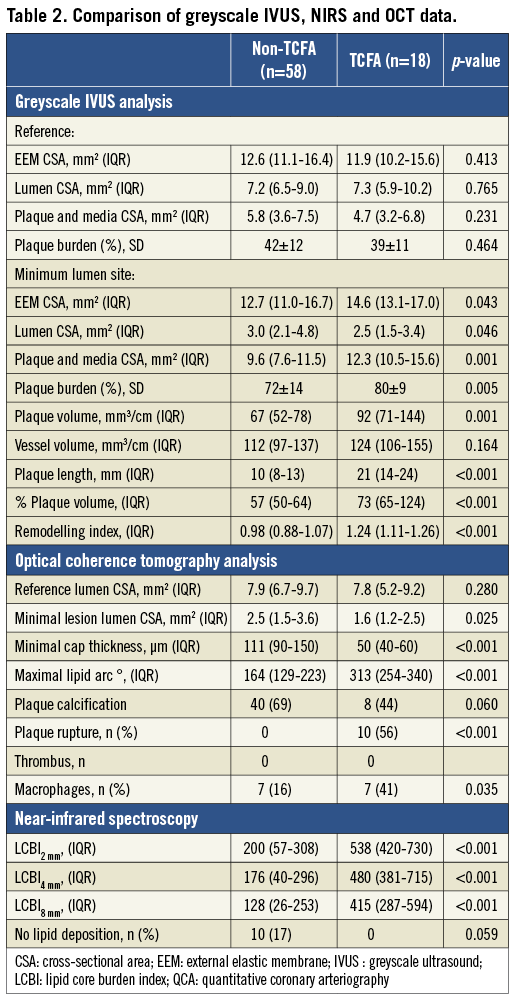
Plaque composition analysis showed that a wider lipid arc and higher maximal LCBI values in each of the 2 mm, 4 mm and 8 mm segment compartments characterised lesions with OCT-defined TCFA. Subclinical plaque rupture, as assessed by OCT, occurred only in OCT-defined TCFA. Data from greyscale IVUS, NIRS and OCT imaging are presented in Table 2.
NIRS DATA AND OCT-DEFINED PLAQUE CHARACTERISTICS
Maximal LCBI values correlated positively with OCT-defined maximal lipid arc (r=0.678 for LCBI2mm, r=0.641 for LCBI4mm, r=0.650 for LCBI8mm; all p<0.001) and negatively with OCT-defined minimal fibrous cap thickness (r=–0.478 for LCBI2mm, r=–0.450 for LCBI4mm, r=–0.489 for LCBI8mm; all p<0.001) (Figure 2).
DIAGNOSTIC VALUE OF NIRS AND IVUS TO CLASSIFY OCT-DEFINED TCFA
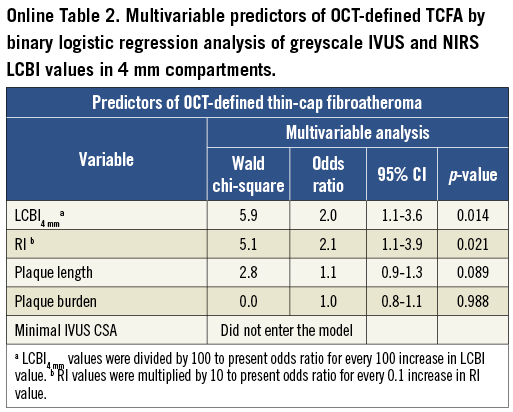
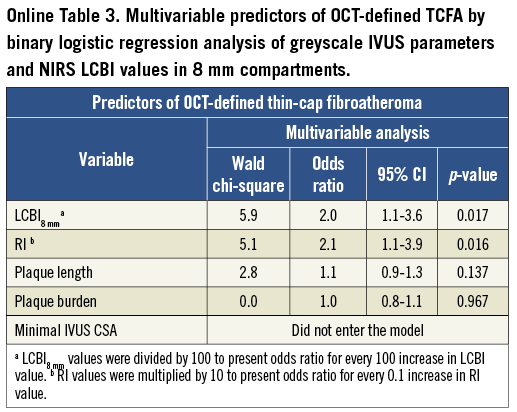
Multivariable logistic regression analysis revealed that LCBI2mm, LCBI4mm, LCBI8mm and RI were independent predictors of OCT-defined TCFA (Table 3, Online Table 2, Online Table 3). Receiver operating characteristic (ROC) analysis was performed for independent NIRS and IVUS predictors of OCT-defined TCFA. The following optimal values for the detection of OCT-defined TCFA were determined in all coronary segments: 315 for LCBI2mm, 265 for both LCBI4mm and LCBI8mm, and 1.046 for RI (Figure 3). The accuracy of IVUS and LCBI criterion values for OCT-defined TCFA detection were then assessed. Sensitivities, specificity, positive and negative predictive values for separate and combined IVUS and LCBI criterion values are presented in Table 4. Figure 3 depicts the accuracy for OCT-defined TCFA detection by LCBI and RI criterion values. We observed the highest accuracy sensitivity and specificity values for LCBI2mm >315 with RI >1.046 as a single combined criterion value, with a sensitivity of 100% and a specificity of 91%. The sensitivity and utility of OCT (cap thickness <65 µm), NIRS (LCBI2 mm) and IVUS (RI) criteria for predicting TCFA and additional potential vulnerable lesions are presented in Figure 4.
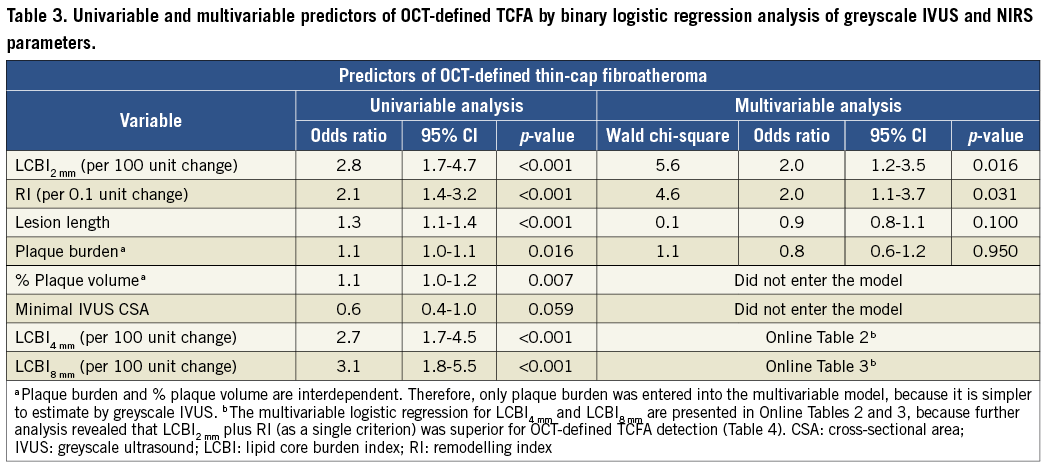
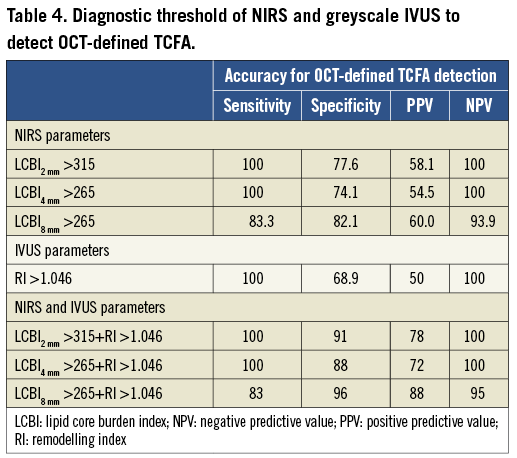
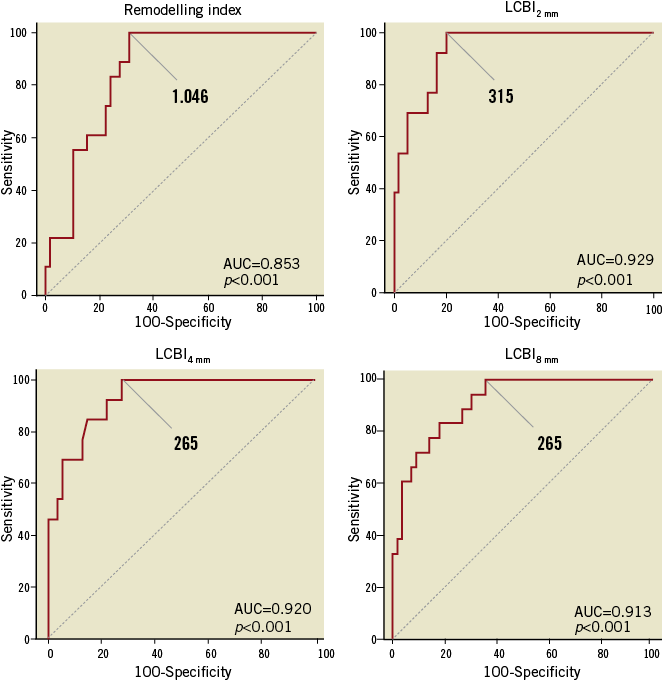
Figure 3. Receiver operating characteristic (ROC) curves of RI and LCBI2 mm, LCBI4 mm, LCBI8 mm for classifying OCT-defined TCFA. Values represent estimated cut-off values for RI and LCBI2 mm, LCBI4 mm, LCBI8 mm. AUC: area under the curve.
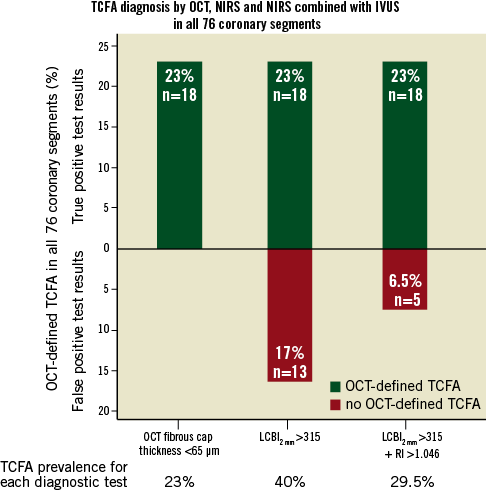
Figure 4. Thin-cap atheroma and potential vulnerable plaque diagnosis for OCT, NIRS and combined NIRS and IVUS in 76 coronary segments. Sensitivity of OCT, NIRS and IVUS for diagnosis of potential vulnerable lesions is shown as number and percentage of lesions (from 76 total assessed lesions). Green columns (above x axis) represent OCT-defined TCFA with cap thickness <65 µm, while red columns (below x axis) represent potential vulnerable lesions that had OCT-defined cap thickness ≥65 µm but either LCBI2 mm >315 alone or LCBI2 mm >315 plus RI >1.046. Note that all lesions with cap thickness <65 µm (shown in green) also had LCBI2 mm >315 and RI >1.046.
INTER-OBSERVER VARIABILITY
The intraclass correlation coefficients for key parameters evaluated in this study were: reference OCT lumen CSA=0.871 (95% CI: 0.703-0.946); maximal lipid arc=0.904 (95% CI: 0.716-0.969); minimal cap thickness=0.909 (95% CI: 0.729-0.971). The kappa for inter-rater agreement for OCT-defined TCFA identification was 0.837 (95% CI: 0.700-0.974).
Discussion
NIRS and greyscale IVUS have previously been used to assess plaque composition in acute coronary settings. These systems are available as a single analysis tool. NIRS has revealed that lesions responsible for acute coronary syndromes are lipid-rich compared to stable coronary lesions18. Greyscale IVUS studies have reported that lesions responsible for future acute coronary events exhibit a higher plaque burden and are associated with positive vessel remodelling19-21. The key finding of our study is that lipid-rich positively remodelled lesions, as assessed by dual NIRS-IVUS imaging, are likely to be thin-cap fibroatheromas.
Our results are of importance for a number of reasons. Firstly, our data extend those from greyscale IVUS6, radiofrequency ultrasound (RF-IVUS)22, integrated backscatter (IB-IVUS)23 and computed tomography imaging24, by demonstrating that positive vessel remodelling, high plaque burden and high lipid burden are associated with OCT-defined TCFA. Our findings are also in accord with a reported association of yellowish colour of the plaque detected by angioscopy with OCT-defined TCFA25,26. Furthermore, the presented prevalence of OCT-defined TCFA is consistent with the previous finding that plaque vulnerability is associated with the severity of angiographic vessel stenosis, plaque burden and positive vessel remodelling4. Moreover, the observed fibrous cap thickness in our study was similar to that observed in a prior OCT-based study in stable coronary patients27. Second, we documented that an increase in lipid core burden assessed by NIRS correlates with decreased minimal fibrous cap thickness assessed by OCT. This LCBI - minimal cap thickness correlation, together with higher LCBI values for OCT-defined TCFA, are in accord with RF-IVUS observations22 and may explain a previous clinical observation regarding target coronary lesions. Madder at al reported generally higher LCBI values in coronary lesions responsible for acute coronary syndromes and lower LCBI values in plaques not responsible for acute syndromes. However, half of the lesions responsible for stable coronary syndromes were also lipid-rich18. In addition, prospective studies have reported that identification of lipid-rich plaque increases the risk of future acute coronary syndromes28,29. Together with these reports, our data beget the important question as to whether some of these lipid-rich but stable plaques may ultimately evolve into TCFA. Third, our results confirmed that the maximal lipid arc assessed by OCT positively correlated with LCBI assessed by NIRS. This complements prior histological validations and demonstrates the validity of both systems for lipid detection and quantification12. Finally, we found that combined analysis of NIRS and greyscale IVUS parameters allows for accurate distinction between stable and vulnerable plaques. While single NIRS analysis was not sufficient for accurate OCT-defined TCFA detection, it was significantly improved by the additional greyscale IVUS analysis of the plaque. The greatest accuracy for OCT-defined TCFA detection was achieved using positive remodelling and LCBI2mm >315. Our findings are consistent with those of IB-IVUS and computed tomography angiography. Miyamoto et al established the utility of IB-IVUS accuracy for OCT-defined TCFA detection in plaques with high lipid core, positive remodelling and plaque burden >75%23. Computed tomography analysis showed that detection of lipid-rich plaque and lesions with RI >1.08 were independent predictors of OCT-defined TCFA24.
Of interest, despite a vulnerable morphological pattern, a small number of lipid-rich and positively remodelled lesions exhibited a fibrous cap thickness of ≥65 µm by OCT. Hence, NIRS and IVUS might detect vulnerable plaques, but without severe thinning of the fibrous cap. Furthermore, prospective computed tomography studies have demonstrated that lipid-rich and positively remodelled lesions resulted in a greater likelihood of subsequent acute coronary syndrome during two years of follow-up28. Therefore, our data raise the provocative question as to whether the current definition of TCFA, being fibrous cap thickness <65 µm by OCT, adequately identifies all vulnerable lesions. However, we note that most ruptured TCFAs silently heal30, and only 4.3% of TCFA were documented to be responsible for major adverse coronary events in three to four years of follow-up29.
Several additional points are raised by our data. In contrast to previous autopsy studies, we did not observe thrombus formation at the site of plaque rupture. However, the study group consisted of stable patients heparinised before imaging, which might dissolve overlying thrombus before OCT imaging. In addition, although controversy has arisen regarding the prevalence of the calcium deposition in TCFAs, our findings are consistent with previous autopsy data31.
Our data are of importance to clinical patient care. Firstly, these findings may help to stratify patients regarding the risk of future acute coronary syndromes and identify vulnerable plaques in the absence of OCT analysis. Thus NIRS-IVUS may also facilitate the assessment of ostial coronary lesions not amenable to OCT analysis, which may be a major advantage of NIRS-IVUS over OCT in terms of vulnerable plaque identification. In addition, by identifying stable lesions (low LCBI and absence of positive remodelling), dual NIRS-IVUS imaging may be used to identify when an invasive treatment might potentially be postponed in favour of medical treatment with aggressive lipid-lowering therapy and other agents. Germane to this possibility, aggressive lipid-lowering therapy has been reported to increase fibrous components of the plaque and decrease plaque vulnerability and lipid content32,33.
Study limitations
The study group consisted of a relatively low number of patients and our findings require validation using a larger sample size. Although OCT is the gold standard for fibrous cap measurement in vivo, it is unclear if this is the optimal definition of a vulnerable plaque34. In addition, NIRS tissue penetration is limited and only provides information about the lipid content at a maximal depth of 1-2 mm into the vessel wall. Hence, NIRS is not informative about the proportion of lipid content in the deeper segments and essentially only provides information about the arc and longitudinal extent of lipid in the coronary vessel12.
Conclusions
OCT-defined TCFA are characterised by positive vessel remodelling, a high plaque burden and greater lipid core burden as assessed by dual NIRS-IVUS imaging. An explicit characterisation of plaque composition might help identify lesions which are responsive to extensive lipid reduction therapy and postpone interventional treatment without adversely affecting clinical outcomes.
| Impact on daily practice Our findings may help to identify vulnerable plaques by combined NIRS-IVUS imaging. It may be a new approach to assess the vulnerability of ostial coronary lesions not reachable for OCT analysis. This is a real advantage of NIRS-IVUS over OCT in terms of vulnerable plaque identification. Moreover, our results show that NIRS-IVUS provides a comprehensive assessment of coronary lesions that estimates their chemical composition, plaque burden and vessel remodelling, and identifies atheroma vulnerability. |
Funding
All subjects in this study were also enrolled in the COLOR registry, which was supported by Infraredx, Inc. Infraredx also provided support for a clinical research imaging fellow who participated in this study. J. Kovacic is supported by National Institutes of Health Grant K08HL111330.
Conflict of interest statement
P. Moreno is a founder and a minor stockholder of Infraredx, Inc., the company that produces the near-infrared catheter used in this study. A. Kini acknowledges honoraria from Medscape and has received research grant support from Infraredx, Inc. S. K. Sharma acknowledges speaker’s honoraria from Boston Scientific, Abbott Vascular, The Medicines Company and Lilly/DSI. The other authors have no conflicts of interest to declare.
Appendix. OCT and NIRS-IVUS image acquisition and analysis, statistical analysis
OPTICAL COHERENCE TOMOGRAPHY IMAGE ACQUISITION
The commercially available C7-XR™ OCT Intravascular Imaging System (OCT C7 Dragonfly™; St. Jude Medical, St. Paul, MN, USA) was used for OCT image acquisition. The tip of the 2.7 Fr OCT catheter was placed at least 15 mm distally to the imaging target lesion. OCT image acquisition was then performed with continuous intracoronary contrast injection (Visipaque™; GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK), total volume 12-16 ml injected at 3-4 ml/sec, and simultaneous OCT catheter pullback at 20 mm/s. Each OCT catheter pullback imaged a total of 54 mm of the vessel.
OPTICAL COHERENCE TOMOGRAPHY IMAGE ANALYSIS
OCT image analysis scrutinised serial cross-sectional images of the vessel, at 1 mm intervals, using the LightLab Imaging Offline Review Station (LightLab Imaging, Inc./St. Jude Medical, Westford, MA, USA). Plaque composition was analysed as follows: signal-rich homogenous plaques were classified as fibrous, signal-poor regions with diffuse borders were classified as lipid, and signal-poor regions with well-defined borders were classified as calcified plaques. Additionally, macrophages were identified as bright spots17. The magnitude of lipid content was measured as the circumferential extent of lipid in OCT cross-sectional images and expressed in degrees (OCT lipid arc). Fibrous cap thickness was derived by measuring the thinnest signal-rich zone separating the lipid content from the vessel lumen (µm). The thinnest part of the fibrous cap was measured three times and its average was defined as the fibrous cap thickness. OCT-defined TCFA was defined as a lipid-rich plaque with fibrous cap thickness <65 µm. In addition, the presence of both plaque rupture and/or luminal thrombus was noted during OCT analysis.
Cross-sectional area (CSA), and minimal and maximal diameter of the vessel were measured every 1 millimetre. The smallest CSA in one segment was taken as the OCT-defined minimal CSA. The OCT reference lumen area was estimated as the largest CSA within 10 mm proximally or distally to the minimal lumen CSA in the scanned coronary segment.
NEAR-INFRARED SPECTROSCOPY AND INTRAVASCULAR ULTRASOUND IMAGE ANALYSIS
COMBINED NIRS AND GREYSCALE IVUS IMAGE ACQUISITION
Combined NIRS and greyscale IVUS image acquisition was performed using the commercially available TVC Imaging System™ with the 2.4 Fr TVC Insight Catheter (Infraredx, Inc., Burlington, MA, USA). The tip of the TVC catheter was positioned at least 10 mm distal to the imaging target lesion. Subsequently, the automated pullback was 0.5 mm/sec (240 rotations/min) until the TVC catheter entered the guiding catheter.
NIRS IMAGE ANALYSIS
The raw spectra of NIRS estimate the probability of the presence of an atherosclerotic lipid core and measurements are displayed as a chemogram – a digit code map. The chemogram is transformed into a colour-coded, red to yellow scaled NIRS map (7-bit), which represents a spectroscopic image of the scanned vessel. The NIRS map’s X-axis indicates the combined NIRS-IVUS pullback position in millimetres, and the Y-axis indicates its circumferential position in degrees. Each pixel on the NIRS map represents the probability of lipid core. Red pixels correspond to a low and yellow pixels to a high probability of lipid core. Pixels with insufficient data (e.g., caused by guidewire shadowing) appear as black.
The NIRS map analysis allows calculation of the lipid core burden index (LCBI). LCBI is estimated by dividing yellow pixels per all pixels (without black ones) within the analysed pullback compartment and are expressed per mill (‰). In the present study, the maximal LCBI was estimated in 2 mm, 4 mm and 8 mm pullback compartments for every analysed lesion (LCBI2mm, LCBI4mm, LCBI8mm). NIRS map analysis was performed using Lipiscan LS107.001rB software (Infraredx).
GREYSCALE INTRAVASCULAR ULTRASOUND IMAGE ANALYSIS
Quantitative greyscale IVUS measurements were performed every one millimetre in scanned coronary segments using QIvus version 2.1 (Medis, Leiden, The Netherlands). Cross-sectional images were quantified for lumen CSA, external elastic lamina (EEM) CSA, plaque and media CSA and plaque burden. Plaque and media CSA were calculated as the difference between EEM CSA and lumen CSA. Plaque burden was calculated as plaque and media CSA divided by EEM CSA x 100 (%) at the minimal CSA site.
The IVUS reference lumen area was estimated as the largest CSA within 10 mm located proximally or distally to the minimal lumen CSA in one analysed coronary segment. The reference EEM CSA was calculated as an average of the proximal and distal EEM CSA with the smallest plaque burden, but not higher than 50%. If either the proximally or distally evaluated plaque burden was ≥50%, then the proximal or distal EEM CSA with the least plaque burden served as the reference.
A lesion was defined as the region between the proximal and distal EEM reference sites or as the region between either the proximal or the distal EEM reference site and a side branch delimiting the assessed coronary segment. Remodelling index (RI) was calculated by dividing EEM CSA at the site of minimal lumen CSA by reference EEM CSA. Lesions with RI ≤0.95 were defined as negatively remodelled, while those with an RI ≥1.05 were defined as positively remodelled. An RI between these values was taken as a non-remodelled vessel.
In every lesion, a lumen vessel volume and EEM volume was calculated based on Simpson’s rule (mm3/cm). These data were used to estimate plaque volume (EEM volume - vessel volume, mm3/cm) and % plaque volume (100 x [plaque volume] / EEM volume, %).
STATISTICAL ANALYSIS
The Kolmogorov-Smirnov test assessed the obtained data distribution. For normally distributed values data are presented as mean with standard deviation (SD), for non-normally distributed values data are presented as median with interquartile intervals (IQR, 25th percentile, 75th percentile). Normally distributed data were compared using Pearson’s correlation and unpaired t-test, while non-normally distributed data were compared using Spearman’s rank correlation and the Mann-Whitney test. The categorical data were compared using Fisher’s exact test or chi-square test.
Univariable and multivariable logistic regression was performed to identify predictors of OCT-defined TCFA. The accuracy of estimated independent predictors for OCT-defined TCFA and the criterion values for NIRS and IVUS measurements were evaluated by receiver operating characteristic (ROC) curves.
The intraclass correlation coefficient and inter-rater agreement (kappa) were evaluated to assess inter-observer variability (MedCalc software version 12.2.1; MedCalc, Ostend, Belgium).