Description
Next-generation OCT (frequency-domain optical coherence tomography, FD-OCT) facilitates high-speed pullbacks during image acquisition without necessitating transient balloon occlusion of the coronary artery1-3.
However, a more robust catheter is necessary to facilitate high-speed pullbacks during FD-OCT. The profile diameter of the FD-OCT catheter is larger than that of the previous generation time-domain OCT (TD-OCT) imaging wire. A larger FD-OCT catheter could occlude severely stenotic lesions, causing insufficient distal contrast flushing. Subsequently, this causes insufficient blood clearance, resulting in poor OCT imaging.
We investigated visualisation challenges of FD-OCT in the presence of severely stenotic lesions in an ex vivo model and developed a new procedure to obtain clear images using FD-OCT (Figure 1 and Figure 2).
Technical specifications
The FD-OCT system (ILUMIENTM; St. Jude Medical, Inc., St. Paul, MN, USA) is composed of an intravascular OCT catheter (DragonflyTM; St. Jude Medical), an imaging engine, a probe interface unit, and a computer console, which also contains a data acquisition board. A 2.7 Fr Dragonfly catheter was delivered as a monorail rapid-exchange catheter over a 0.014-inch coronary guidewire through a 6 Fr guide catheter. After catheter placement, a cardiovascular injection pump was used to deliver the contrast medium through the guide catheter at a rate of 4 ml/sec for a total of 14 ml or 3.5 sec. The FD-OCT system performed pullback of the imaging catheter optics at a rate of 20 mm/sec. Our protocol allowed imaging of approximately 5 cm of the coronary segment over a period of 3.5 sec with 14 ml of the contrast medium1-3.
Indications for use
This new procedure should be used for FD-OCT imaging of severe stenosis in which the conventional procedure would fail to yield images.
Tips and tricks for use
After passing a guidewire through the target lesion, we confirmed that the Dragonfly catheter could pass through the lesion without problems. Once this was confirmed, the Dragonfly catheter was retracted to a position proximal to the target lesion. The pullback trigger of the FD-OCT system was set to manual, the system mode switched to live view and pullback enabled.
Once the system settings were prepared, the contrast medium was injected into the target coronary artery (left coronary artery: flow rate 4 ml/sec, volume 14 ml; right coronary artery: flow rate 3 ml/sec, volume 12 ml) as per the manufacturer’s instructions. The Dragonfly catheter was passed through the target lesion and, as soon as positioning was complete, pullback was initiated. When pullback was complete, contrast flush was discontinued and the Dragonfly catheter retracted into the guide catheter.
Preclinical experience
We successfully acquired clear images using the new procedure in our ex vivo model. It was difficult to acquire clear images using the conventional procedure (Figure 1 and Figure 2).
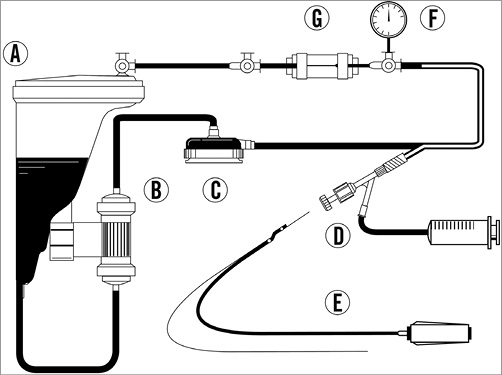
Figure 1. Circuit diagram of the ex vivo model used in the simulation of the new procedure. A) A reservoir of circulating fluid contained 2 l saline, 1.4 l glycerol and red dye. Ratio of circulating saline to glycerol was 10:7 and circulating fluid was maintained at 20°C. Fluid viscosity was equal to blood analogue at 4 cP. B) A low-temperature heat exchanger maintained the liquid at 20°C. C) A centrifugal pump maintained the internal pressure at 50-80 mmHg and the circulation velocity at 100-150 ml/min. D) A coronary guidewire and Dragonfly catheter were inserted via a 6 Fr sheath and 6 Fr guiding catheter, and the contrast medium was injected by a cardiovascular injection pump. E) The Dragonfly catheter and a guidewire. F) An internal pressure gauge monitored internal pressure. G) A stenotic lesion 1 cm in length and with an internal diameter of 3.4 Fr was created in the mock vessel.
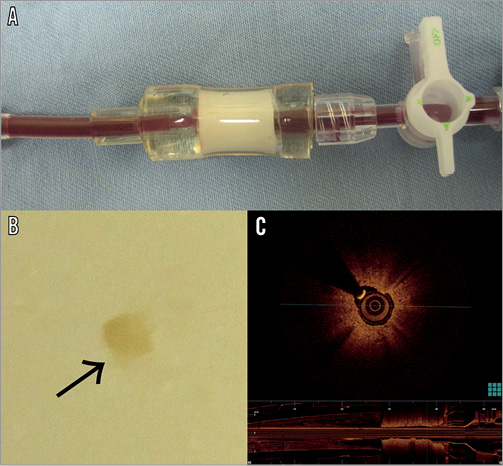
Figure 2. A) A stenotic lesion 1 cm in length in the mock vessel made of boiled fish-paste. B) A section of the stenotic lesion within the mock vessel with an internal diameter of 3.4 Fr (arrow). C) An image segment, acquired using optical coherence tomography, of the stenotic lesion in the mock vessel.
Clinical experience
In our hospital, OCT was used in ACS patients primarily to observe the culprit lesion.
We performed FD-OCT in 109 patients (of whom 92 exhibited ACS) between December 2011 and September 2012. OCT imaging was attempted in 116 patients, but the catheter could not cross the lesion in seven. These seven patients were not included in the analysis.
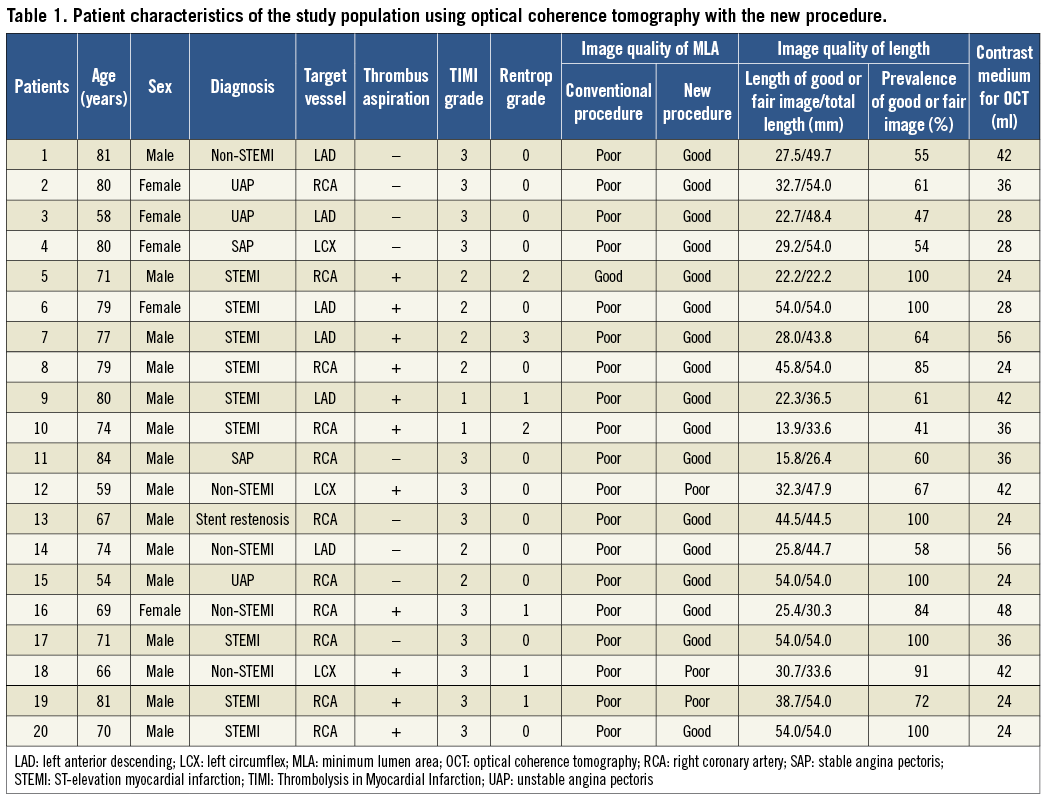
In patients with TIMI 0-1 coronary flow after guidewire crossing, we attempted to obtain TIMI 2 flow. However, if this manoeuvre failed to guarantee an adequate anterograde coronary flow, direct thrombus aspiration was performed before imaging. The conventional procedure failed to acquire clear images in 19 patients. We performed the new procedure in 20 patients (17 with ACS, two with stable angina pectoris and one with stent restenosis) (Table 1). This group comprised 19 patients in whom the conventional procedure failed to acquire clear images and one patient who was used for image comparison.
We successfully acquired clear images in 17 patients using the new procedure (Figure 3, Moving image 1-Moving image 3). In the three remaining patients, because of inadequate blood clearance distal to the lesion, OCT signal attenuation due to blood flow was observed.
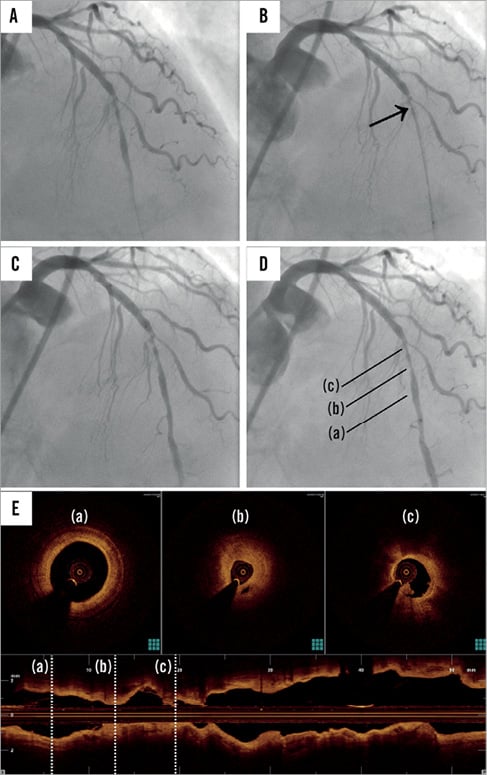
Figure 3. Successful coronary angiography and optical coherence tomography in one of 17 patients (Table 1, patient 6) achieved using new procedure. A) Angiographic image of the coronary guidewire crossing a lesion in the left anterior descending artery. B) Optical coherence tomography (OCT) image of the catheter positioning using the conventional procedure. The contrast medium was injected using a Dragonfly catheter positioned through the stenotic lesion. Images could not be acquired distal to the lesion. Flow was occluded by the catheter wedged at the lesion (arrow) (Moving image 1). C) & D) OCT images of the catheter positioning using the new procedure. Clear images were acquired because pullback was initiated after crossing the lesion while the contrast medium was continuously injected (Moving image 2). E) OCT image of the new procedure: clear image segments show (a) the distal side of the lesion, (b) the stenotic lesion, and (c) the proximal side of the lesion.
Qualitative measurements assessed in this study included visibility of the lumen border at the minimum lumen site and visibility of the length of the observation site. The visibility of the lumen border by OCT was classified into three grades: (1) good (entire circumference visible), (2) fair (>75% of circumference visible), and (3) poor (<75% of circumference visible) (Figure 4)4. These grades ignore the wire artefact. The length of these grades was also measured. The prevalence of good/fair grade images was determined by dividing the length of good/fair images by the total length of observation (excluding guiding catheter) (Table 1). There was no significant difference in the length of clear image between the conventional (n=77, 34.5±11.0 mm) and the new procedure (n=20, 33.4±12.8 mm; Student’s t-test, p=0.64). The difference between the control group (good or fair=29 [70%], poor=12) and post-adoption of the new procedure (good or fair=65 [95%], poor=3) was not statistically significant (chi-square test, p=0.001).
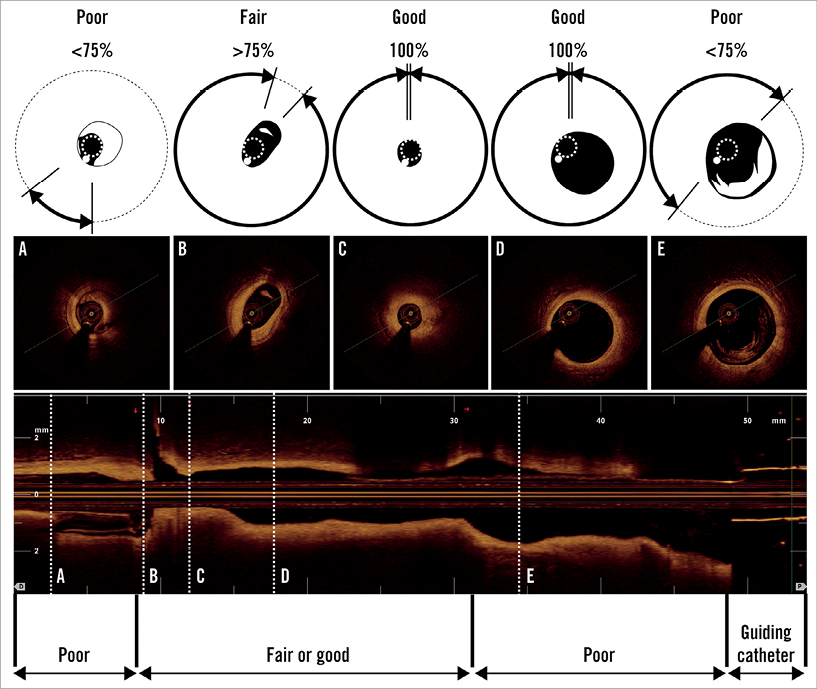
Figure 4. Grading of visibility of the lumen border in OCT imaging. Good=visible on entire circumference. Fair ≥75% of circumference. Poor ≤75% of circumference. A) Poor image: distal site of lesion. B) Fair image: distal site of lesion. C) Good image: lesion. D) Good image: proximal site of lesion. E) Poor image: proximal site of lesion.
Patient 7 (Table 1) exhibited grade 3 Rentrop collateral flow, and we could not acquire clear images using the conventional procedure5.
Finally, no complications such as acute vessel occlusion, dissection, significant arrhythmias or vasospasm along the entire procedure-related artery were associated with this new procedure.
Discussion
Our new FD-OCT procedure acquired clear images of severely stenotic lesions, something which is difficult using the conventional procedure. The conventional procedure failed to acquire clear images because the catheter itself occludes the coronary artery when traversing a severe stenosis, preventing the contrast medium from flushing out the blood for clear imaging.
In the case of patient 7 (Table 1), who exhibited grade 3 Rentrop collateral flow, imaging was clear using the new procedure. In this case, anterograde flow competed with retrograde flow. However, anterograde flow was overcome by retrograde flow when the Dragonfly catheter crossed the target lesion in accordance with the “Dotter effect”6.
Performing the new procedure requires specific techniques for both the Dragonfly catheter operator and the system console. Precise timing of contrast medium flushing and pullback initiation is crucial to achieve clear images. Practice with our ex vivo simulator allowed us to learn the steps that are key to the success of the new procedure and apply them smoothly in the clinical setting. These steps include prior confirmation that the catheter can cross the lesion, selection of a location for catheter placement and indication by the operator that the technician should initiate pullback as soon as the catheter has crossed the lesion.
In three patients in whom the new procedure failed, blood remained distal to the lesion despite the fact that the lesion itself was flushed (Table 1, patients 12, 18, & 19). We think these failures resulted from operators who had not practised with the ex vivo simulator and mismatched the start time and contrast medium flush settings. Further investigation allowed for the optimisation of the flush rate and the timing of contrast medium injection.
In addition to conventional flushing through the guiding catheter, another technique that uses simultaneous injection of contrast media through the distal port of the imaging catheter (gentle flushing) exists7. While this technique may yield acceptable images, using the narrow imaging catheter purge lumen in this manner creates pressure that distorts live OCT imaging and may introduce the risk of imaging core breakage while the optics are turning at high speeds. Both procedures offer effective alternatives when conventional flush is not an option; however, our technique may limit the time that the OCT catheter occludes the coronary artery.
There are two limitations of the new procedure. First, the Dragonfly catheter has to traverse the target lesion quickly. Hence, this procedure is unsuitable for complicated lesions. Second, as with the conventional procedure, the new procedure requires a contrast medium injection and carries the risk of contrast-induced nephropathy3,8,9. Low-molecular-weight dextran can be used instead of contrast medium, but this makes it difficult to visualise whether blood is adequately flushed3.
The new procedure effectively and safely obtained clear images in coronary arteries in which the conventional procedure failed to achieve successful visualisation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Coronary angiography of conventional procedure.
Moving image 2. Coronary angiography of new procedure.
Moving image 3. Optical coherence tomography of new procedure.
Supplementary data
To read the full content of this article, please download the PDF.

