Abstract
Aims: Optical coherence tomography (OCT) allows a detailed assessment of intimal coverage and strut apposition which are well known substrates for late thrombosis. This study sought to assess and compare long-term coverage and apposition of PES and EES implanted in different lesions of the same coronary artery (and in the same patient).
Methods and results: A total of 30 patients were included. In these patients PES and EES were implanted in the same vessel in two similar lesions. The selection of the stent for each lesion was random. At 12 months, 30 PES were examined analysing 154±90 struts/stents and 30 EES analysing 158±72 struts/stents. The proportion of uncovered struts was 0.8±1.3% for EES and 1.5±2.9% for PES (p=0.3), and the proportion of malapposed struts was 1.25±2.1% and 0.98±2%, respectively (p=0.2). A pooled analysis was performed using the random effects model, given the significant heterogeneity found, which did not show significant differences between EES and PES for non-coverage (RR 0.73, 95% CI: 0.32-1.67) or malapposition (RR 1.60, 95% CI: 0.56-4.61). The presence of non-coverage in malapposed struts was 62% with PES and 15% with EES (p<0.0001), the maximal malapposition area being significantly larger with PES (0.6±0.3 vs. 0.25±0.2 mm2, p=0.001).
Conclusions: In highly matched conditions, with PES and EES implanted in the same artery, both DES showed a comparable degree of intimal coverage and apposition at one-year follow-up. A smaller area of malapposition with non-covered struts was found with EES.
Abbreviations
BES: biolimus-eluting stent
BMS: bare metal stent
DES: drug-eluting stent
EES: everolimus-eluting stent
IVUS: intravascular ultrasound
OCT: optical coherence tomography
OFDI: optical frequency-domain imaging
PES: paclitaxel-eluting stent
SES: sirolimus-eluting stent
ZES: zotarolimus-eluting stent
Introduction
Drug-eluting stents (DES) have been related to a variable but constant degree of very late thrombosis. Incomplete intimal coverage and late acquired malapposition have been the most important mechanisms involved following in vivo ultrasonographic studies and necropsy studies1-3.
Optical coherence tomography (OCT), with an axial resolution of 10-20 µm (higher than that of IVUS), allows a detailed assessment of intimal coverage and strut apposition4,5. Clinical trials and some registries available to date have suggested a lower late thrombosis rate with second-generation DES, especially everolimus-eluting stents (EES), but these trials were underpowered to draw definitive conclusions regarding the specific endpoint of late thrombosis6,7. Moreover, differences observed between different generations of DES regarding stent thrombosis have been pointed out in the subacute period, but no conclusions can be established concerning late and very late thrombosis8,9.
Using OCT, this study sought to assess and compare long-term intimal coverage and stent apposition in first-generation DES (paclitaxel-eluting stent [PES] TAXUS® Liberté®; Boston Scientific, Natick, MA, USA) versus second-generation DES (everolimus-eluting stent [EES] XIENCE PRIME™; Abbott Vascular, Santa Clara, CA, USA) implanted in different lesions of the same coronary artery (and in the same patient).
Methods
STUDY POPULATION
During 2009 we prospectively included all patients referred for coronary angiography for known or suspected coronary artery disease as long as they met all the following inclusion criteria:
a) clinical indication of revascularisation;
b) two different de novo lesions in the same vessel within a distance sufficient to allow the deployment of two non-overlapped stents (>20 mm length between lesions);
c) lesions with similar morphological features; maximal length difference of 5 mm and reference diameter difference of less than 1 mm; lack of angiographic signs of plaque rupture or thrombosis; ostial, bifurcated and total occlusion lesions were excluded;
d) index procedure without significant complications (such as dissection, haematoma or acute thrombosis);
e) signed patient consent form.
The exclusion criteria were the following:
a) contraindications for long-term dual antiplatelet treatment (such as high bleeding risk, scheduled surgery or oral anticoagulation);
b) primary angioplasty procedure.
The selection of the stent for each lesion was performed by means of a computer-generated random sequence. In those procedures in which IVUS was used, it helped to optimise the results of both stents. Implantation pressure and post-dilatation, being decided by the operator, had to be similar for both stents.
The study was approved by the local research ethical committee. Informed consent was obtained from all patients included in this study.
FOLLOW-UP STUDY
One-year angiographic and OCT follow-up was scheduled. Those patients who were going to require an angiogram during the first year of follow-up due to symptoms were planned to be excluded. OCT analysis was performed on a blind basis by two different investigators. Two analyses strut by strut were carried out: first, a separate analysis by each investigator, and a second one performed by both. Discrepancies were solved by consensus involving a third investigator.
OCT ACQUISITION AND ANALYSIS
We used an optical frequency-domain imaging system (FD OCT), Model C7 Cardiology Imaging System (LightLab Imaging, Westford, MA, USA). The monorail imaging catheter was advanced distal to the stented segment. During image acquisition (20 mm/sec pullback speed) with a non-occlusive technique, coronary blood was displaced by automatic infusion of contrast (Visipaque, Iodixanol 320; GE Healthcare, Cork, Ireland). The optimal volume/time intracoronary infusion of contrast was tested to achieve a complete blood clearance in the vessel lumen.
Off-line analysis of the cross-sections within the stented segment was performed at 1 mm intervals. The strut apposition and neointimal coverage were assessed for each strut. Interobserver variability was assessed in two stents (n=302 struts), one of each stent type, measured by two different analysts.
Assessment of coverage: distances (neointima thickness) between the luminal surface of the neointima and stent strut were measured manually, aligned perpendicularly to the strut. The strut was classified as uncovered if any part of the strut was visibly exposed to the lumen with no layer of tissue overlying its bright signal-intense structure.
Assessment of apposition: distances between the luminal edge of the strut reflection and the vessel wall were measured perpendicularly to the strut. Stent malapposition was defined as struts with detachment from the vessel wall over the total strut thickness (metal strut+polymer) for each stent type, TAXUS® Liberté® (metal strut 97 µm and polymer 15 µm) and XIENCE PRIME™ (metal strut 81 µm and polymer 8 µm). Bifurcational cross-sections were excluded from this analysis. The maximal area of malapposition was obtained in every case. This comprised an area between a line traced following the abluminal surface of the malapposed struts and a line traced over the adluminal surface of the vessel wall with both lines merging at the level of the adjacent apposed struts.
Other measurements obtained in every stent were the maximal intimal area and thickness, the minimum stent area, the minimum in-stent lumen area and the in-stent area stenosis.
STATISTICAL ANALYSIS
The study was exploratory in nature and no formal sample size was calculated. Continuous variables are presented as mean ± standard deviation. Categorical variables are expressed as percentages. Continuous variables were compared with the Student’s t-test if the data followed a normal distribution and with Wilcoxon tests if the data were skewed. Categorical variables were compared with the χ2 test or the Fisher’s exact test, where indicated. All probability values were two-sided and values of p<0.05 were considered statistically significant. The risk ratios (RR) for non-coverage and for malapposition of EES vs. PES were calculated by means of pooled analysis using an inverse variance random effects model, taking into account the between-clusters and within-the-cluster variability, using each pair of matched stents as an independent unit of clustering10. Those pairs of stents with both devices completely covered were not considered informative for evaluation of the RR for non-coverage and so were discarded from the analysis. Likewise, those pairs with both devices completely apposed were considered not informative to evaluate the RR for malapposition and hence discarded10. A proportional continuity correction was applied to stents with zero uncovered struts (zero events) in only one of the stents. The risk ratio of each individual stent and the pooled risk ratio of the whole sample were graphically represented by means of forest plots. Analysis of heterogeneity of the effect was reported as I2 (proportion of the effect attributable to heterogeneity) and the p-value of the Q-test, a p-value of ≤0.1 being considered statistically significant. Interobserver variability is reported as Cohen’s kappa test for the primary outcome (binary coverage) or as intraclass correlation coefficient for the absolute agreement (ICCa) in continuous variables (thickness of coverage). Calculations for pooled analysis were performed with the CMA version 2 (Biostat Inc., Englewood, NJ, USA) software package. For the remaining tests the statistical package SPSS 15.0 (SPSS Inc., Chicago, IL, USA) and MedCalc 9.6 (MedCalc Software, Ostend, Belgium) were used throughout.
Results
From January 2009 to December 2009, 30 patients, whose clinical characteristics are described in Table 1, were included in the study. The clinical profile of these patients corresponds to a medium- to high-risk population according to the required multilesional condition. Target lesions were more frequently located in the left anterior descending artery.
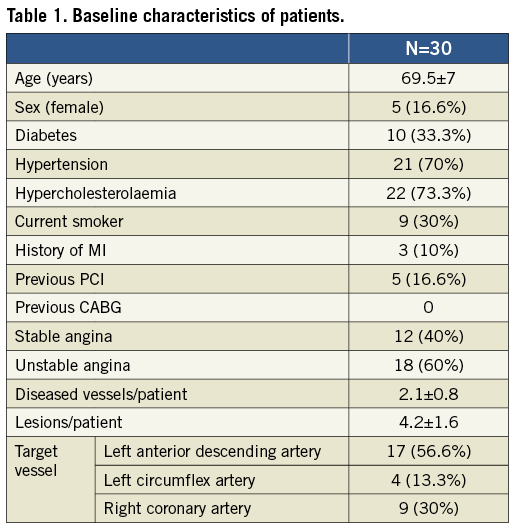
These patients were treated in the same artery with both stent types in two separate but similar lesions. The angiographic characteristics of the lesions treated with the different stents are presented in Table 2. There were no significant differences between groups regarding angiographic measurements and procedural data. The use of IVUS and post-dilatation as well as the maximal implantation pressure were all quite similar.
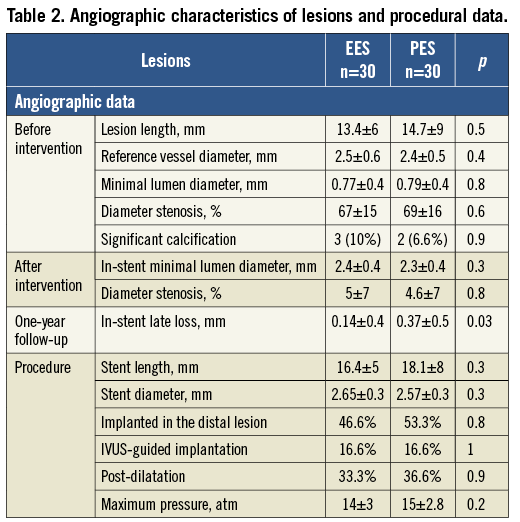
By one-year follow-up no major cardiac adverse events had occurred, and in all 30 patients the angiography was performed 12 months after the procedure as planned per protocol. Angiographic analysis showed significantly less late loss in EES (Table 2).
The findings with FD OCT are presented in Table 3. A comparable number of struts was analysed in each group. The amount of intimal proliferation was larger with PES, and correspondingly the in-stent minimum lumen area was lower and the in-stent area stenosis was higher in this group, these differences all being significant. The proportion of struts without coverage or with malapposition did not differ significantly between groups. The pooled analysis was conducted for non-coverage (excluding the 11 pairs with full coverage) and for malapposition (excluding the 15 pairs with complete apposition). The random effects model is reported given the significant heterogeneity observed. This analysis did not yield significant differences between both stents for non-coverage or malapposition (Figure 1, Figure 2). However, it is interesting to note that the presence of non-coverage in malapposed struts was 62% with PES and 15% with EES (p<0.0001).
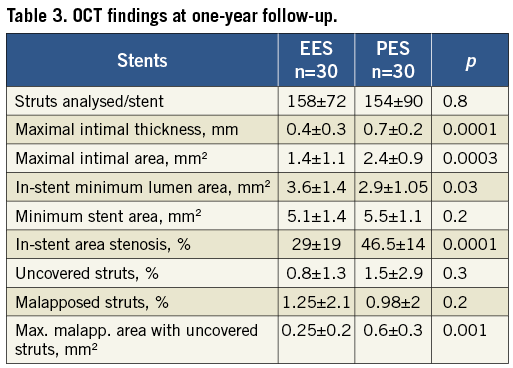
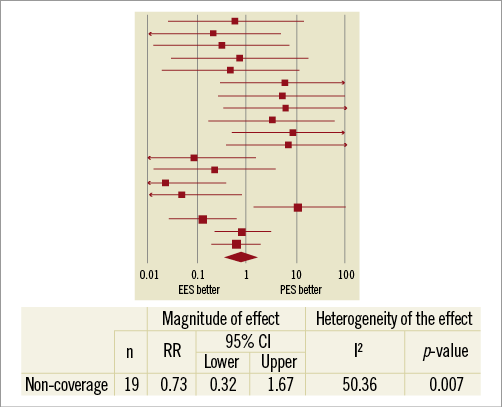
Figure 1. Pooled analysis of the risk ratio of non-coverage in EES vs. PES.
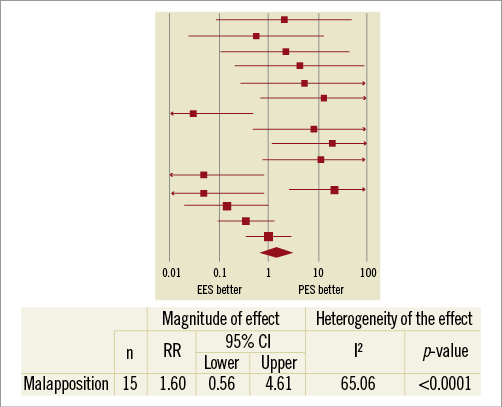
Figure 2. Pooled analysis of the risk ratio of malapposition in EES vs. PES.
In Figure 3 some case examples of good coverage, non-coverage and malapposition (either isolated or in combination) are presented. There was good interobserver agreement in the assessment of binary coverage (kappa=0.684, p<0.0001) and excellent agreement in the measurement of thickness of coverage (ICCa=0.950, 95% CI: 0.937-0.960).
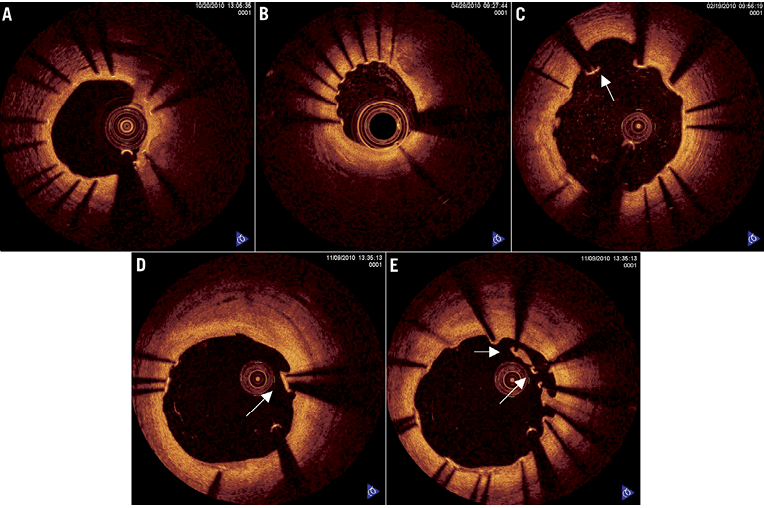
Figure 3. A) Stent cross-section with all struts showing full intimal coverage. B) Cross-section of one stent with non-covered but well-apposed struts. C) Non-covered and malapposed struts. D) Covered and malapposed struts. E) Malapposed struts with and without coverage.
Discussion
In this study we have been able to show that, in equivalent conditions, both XIENCE and TAXUS DES have a similar, large one-year intimal coverage. It is of interest to note that significant heterogeneity was observed despite the fact that both DES were implanted in the same patient. On the other hand, the presence and extent of malapposition among uncovered struts was greater with PES. In addition, the degree of intimal proliferation was greater with PES, leading to a statistically significant larger late lumen loss. This apparent paradox, PES having more uncovered struts while also having more late loss and more intimal proliferation, could be explained by the fact that most of the non-coverage with PES was found in malapposed struts, conversely to EES. The malapposition rate was not statistically different, but with PES the magnitude was greater and more frequently associated with non-coverage.
The larger areas of incomplete apposition observed with PES, the more frequent non-coverage of PES malapposed struts and, simultaneously, the thicker intimal proliferation with PES suggest a potentially higher prevalence of late acquired malapposition with these stents. Nevertheless, this is a hypothetical consideration. A recently published study has shown a more delayed coverage of malapposed and non-apposed side-branch struts with respect to well-apposed struts10. The deficient coverage also observed in side-branch struts supported the hypothesis that incomplete apposition at the time of implantation was the main reason for non-coverage. However, this study was carried out with “-limus drug”-eluting stents. The mechanisms underlying the association between malapposition and non-coverage could not be equivalent with paclitaxel-eluting stents, as is suggested by our results.
Given the fact that the very late thrombosis rate is very low and is scattered in time, it becomes a challenge to design a clinical trial powered to point out differences in the very late thrombosis rate between different DES designs6,9. Registries have the advantage of including large seri es and longer follow-ups, but their accuracy to compare stents is very limited, even after statistical adjustments11,12.
From necropsy studies we know that the most relevant findings observed in late and very late thrombosis in DES are the lack of intimal coverage and late incomplete apposition1,2. In vivo IVUS studies have related large acquired incomplete apposition to very late thrombosis3, but it has not been possible to assess intimal coverage with this technique due to its spatial resolution (100-150 µm). Strut coverage could be an important morphometric predictor of late stent thrombosis. In vivo assessment of stent coverage requires an imaging technology with adequate spatial resolution. A pathological study has shown that a high proportion (about 64%) of sirolimus-eluting stents were covered by a thin intimal layer <100 µm in thickness, which is beyond IVUS resolution13.
Intravascular OCT, using infrared back-reflected light, provides a greater resolution (10-20 µm) and fewer strut-induced artefacts, which facilitates a more refined analysis of strut coverage and apposition. This imaging modality could nowadays be considered the best tool to assess strut coverage and apposition4,5,10,14-16. OCT studies have shown that first-generation DES can present with a certain lack of intimal coverage even two years after their implantation17,18. The presence of incomplete apposition with sirolimus DES has been related to lower intimal coverage and the presence of thrombus19.
A limited number of studies involving second-generation DES suggest a wider degree of intimal coverage. Accordingly, the ENDEAVOR OCT study revealed an almost complete intimal coverage and no incomplete acquired apposition at three months with zotarolimus-eluting stents (ZES) (Endeavor® Sprint; Medtronic, Minneapolis, MN, USA)20. The same research group compared these stents to sirolimus-eluting stents, remarking on a lower extension of intimal coverage, higher incomplete apposition and more thrombus in the latter21. Inoue et al studied 25 patients with EES eight months after implantation: 98.4% of struts were covered and only 0.54% showed malapposition22.
In order to compare different models of DES by means of OCT, the most suitable design would be that of a randomised study with a large population as in the HORIZONS-AMI study (with 118 patients with PES or bare metal stents)23. However, there have also been other studies with a lower number of patients involved, such as the OCTAMI study (44 patients with ZES or BMS) and the LEADERS substudy with 46 patients with biolimus-eluting stents (BES) or SES24,25. The assessment and comparison between stents with OCT has also been performed in a particular clinical subset, such as myocardial infarction, either in registries or clinical trials23,24,26. These studies have revealed a greater proportion of non-covered struts with PES vs. BMS in the setting of a myocardial infarction (at 13 months: 5.7% vs. 1.1%, p<0.001)23, a similar proportion between BMS and ZES in the same subset (at six months: 0% vs. 2%)24, and a lower proportion with BES vs. SES in different clinical settings (at nine months: 0.7% vs. 2.7%)25. A non-randomised study compared three different models of DES in 46 patients with myocardial infarction at nine months, the best intimal coverage being with ZES, followed by PES, and leaving SES in last place26. There are also studies that compare the same stent in different clinical settings, such as ST-elevation myocardial infarction, unstable and stable angina27. OCT has also been used to assess different stents in particular lesion subsets, such as bifurcations or left main disease28,29.
The implantation of different stents in different lesions of the same coronary artery provides an analogous anatomic and biological subset for comparison of the stents. Thus, we consider this design to have great value in comparing the performance of different stents. This methodology has already been used in a study that compared PES and SES in 27 patients. Both stents were deployed in the same artery, and OCT was performed six months later. The authors found a larger but more irregular intimal coverage with PES30.
Our outcomes are concordant with previous findings in animal studies where EES endothelialisation was quicker compared to other durable polymer DES. However, those were animal studies at 14 and 28 days31. This better performance of EES could be explained in different ways. First of all, it might be influenced by the thinness of both the metallic strut and the polymer32. Next, the fluorinated polymer has demonstrated a faster rate of endothelialisation contributing to long-term healing. The modulation of host-material interface by a fluorinated surface, combined with the chemical stability of the fluorinated copolymer, elicits a cellular response conducive to healing with minimal chronic inflammation33,34. Last, the different cellular and molecular actions of the macrocyclic lactone group (“-limus drugs”) and paclitaxel, as well as the different release kinetics, might play a role35.
Limitations
The number of patients is limited. Although we have compared stents implanted simultaneously in different lesions of the same coronary artery with a randomised localisation of the stents, the lesions were not completely comparable in both groups. The presence or lack of intimal coverage is limited to the spatial resolution of the technique, in such a way that an intimal layer of less than 10 µm could not be detected. We cannot assess whether the malapposition detected in follow-up studies was acquired or not, not having performed an OCT immediately after implantation in the index procedure. The OCT analysis used in this study is affected by some limitations as compared to other methodological approaches25; however, it still remains as a valid approach to assess the healing process after DES implantation. The OCT analysis was focused on a strut-by-strut (every 1 mm) observation. The technique does not systematically provide perfect images and “borderline” findings can be seen.
Conclusions
In very similar conditions, no significant differences were observed in intimal coverage and strut apposition between PES and EES at one-year follow-up. As a secondary finding, EES showed a smaller area of malapposition with uncovered struts.
Conflict of interest statement
The authors have no conflicts of interest to declare.

