
Abstract
Aims: Peri-strut low-intensity area (PLIA) assessed by optical coherence tomography (OCT) has been reported as a potential marker of abnormal neointimal healing. We aimed to evaluate the impact of PLIA on clinical events and its risk factors.
Methods and results: We enrolled 264 consecutive patients treated with an everolimus-eluting stent (EES) who underwent follow-up OCT six to 12 months after stenting. Target lesion revascularisation (TLR) was evaluated at a mean 42.6 months after stenting. PLIA was identified in 102 patients; 162 patients did not exhibit PLIA. Multivariate Cox hazard regression analysis indicated that the presence of PLIA (PLIA+) was an independent risk factor for an increased incidence of TLR (hazard ratio [HR]: 4.608, p=0.003). In both the early (<1 year) and late (>1 year) phases, the incidence of TLR was significantly higher in the PLIA+ group (p<0.001 and p<0.001, respectively). In the Cox hazard regression analysis, current smoking and increased C-reactive protein level were independently associated with PLIA+ (HR: 1.737, p=0.009; HR: 2.435, p=0.008, respectively).
Conclusions: The presence of PLIA on midterm OCT was associated with TLR after EES implantation. Detailed stent assessment by midterm OCT may help to predict stent failure in patients treated with EES.
Abbreviations
ACS: acute coronary syndrome
CAD: coronary artery disease
CRP: C-reactive protein
DES: drug-eluting stent
EES: everolimus-eluting stent
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
PLIA: peri-strut low-intensity area
TLR: target lesion revascularisation
Introduction
Optical coherence tomography (OCT) has played an integral role in the quantitative and qualitative analyses of the neointima after stenting. The presence of a low-intensity area surrounding the stent struts, referred to as peri-strut low-intensity area (PLIA), has been demonstrated to correspond to the presence of fibrinoid and/or hyaline extracellular protein in swine1. We previously reported a potential linkage between the presence of PLIA on midterm follow-up OCT images and neointimal growth in patients with first-generation drug-eluting stents (DES)2; however, the risk factors for PLIA and its clinical implications have yet to be elucidated. Therefore, this study aimed to clarify the potential risk factors of PLIA and evaluate its effect on clinical events in a large Japanese observational OCT database of patients with coronary artery disease (CAD).
Methods
PATIENT POPULATION
The Kobe University Hospital OCT registry is a single-centre registry of consecutive patients who underwent OCT of the coronary arteries between April 2011 and March 2015. During this period, OCT was performed because of 1) planned follow-up coronary angiography and OCT for routine stent follow-up or for other study protocols, regardless of symptoms, and 2) evidence of myocardial ischaemia such as silent myocardial ischaemia, stable angina, or acute coronary syndrome (ACS). Patients enrolled in the registry who met the following criteria were included: 1) treatment with everolimus-eluting stents (EES) (XIENCE V®; Abbott Vascular, CA, USA, or PROMUS Element™; Boston Scientific, Marlborough, MA, USA), and 2) follow-up OCT examination six to 12 months after stenting. Among these patients, we excluded those with 1) a stent implanted in the left main trunk, or 2) insufficient quality of OCT images. For patients with stents implanted in more than two lesions, we selected the most recently implanted stent as the target. Additionally, among patients who were implanted with multiple stents in tandem in the same vessel at the index procedure, we selected those with a proximal stent for analysis. On follow-up OCT images, PLIA was defined as a region around the stent struts with a more homogenous lower-intensity appearance than the surrounding tissue without significant signal attenuation behind the area (Figure 1A)2. Patients were divided according to the presence or absence of PLIA (PLIA+ and PLIA– groups, respectively). Percutaneous coronary intervention (PCI) was performed under intravascular ultrasound (Boston Scientific, or Volcano Corporation, Rancho Cordova, CA, USA) or OCT guidance (C7 Dragonfly™ or C8 Dragonfly™; St. Jude Medical, St. Paul, MN, USA). All patients were receiving aspirin (100 mg/day). Patients with stable angina received ticlopidine (200 mg/day) or clopidogrel (75 mg/day) for at least three months; those with ACS received at least six months of ticlopidine or clopidogrel after stenting.
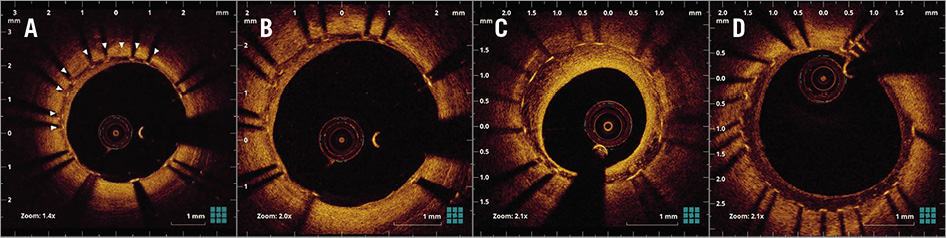
Figure 1. Representative OCT images of peri-strut low-intensity area (PLIA) and PLIA score. A) White arrowheads indicate PLIA. B) PLIA score 1: PLIA that proliferated only around stent struts. C) PLIA score 2: PLIA observed in part of the neointima. D) PLIA score 3: PLIA observed in the entire neointima.
This study was approved by the ethics committee of Kobe University and performed according to the guidelines of the Declaration of Helsinki. All patients provided written informed consent.
OCT EXAMINATION AND ANALYSIS
OCT examination was performed using frequency-domain OCT systems as previously reported3. Offline OCT analysis was performed using dedicated software (LightLab Imaging Inc., Westford, MA, USA). All frames were analysed by independent investigators blinded to angiographic and clinical findings. When PLIA was detected in at least one, the patient was considered to be PLIA+. For the quantitative evaluation of PLIA, PLIA scores were defined as follows: 1=PLIA that proliferated only around the stent struts; 2=PLIA observed in part of the neointima; 3=PLIA observed in the entire neointima (Figure 1B-Figure 1D). To quantify the prevalence of +PLIA struts, the incidence of +PLIA struts was calculated as the number of +PLIA struts/number of all evaluated struts (%) for each stent2. Neoatherosclerosis was defined as neointima containing a diffuse border and a signal-poor region, with rapid signal attenuation. In-stent thrombus was defined as a mass protruding beyond the stent strut into the lumen, with significant attenuation behind the mass. Vasa vasorum was defined as well-delineated low backscattering structures <200 μm in diameter showing a trajectory within the vessel (Figure 2A-Figure 2C)4. Stent underexpansion was evaluated using follow-up OCT images with minimal stent area <80% of the mean of proximal and distal vessel reference area5.
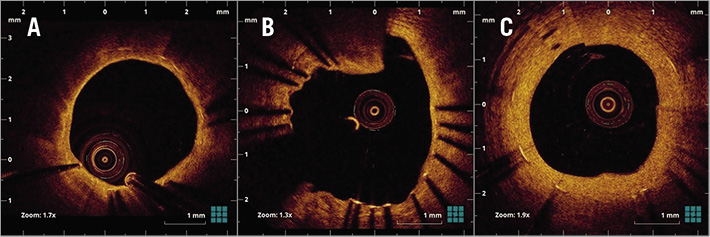
Figure 2. Representative optical coherence tomography images. A) Neoatherosclerosis. B) In-stent thrombus. C) Vasa vasorum.
REPRODUCIBILITY OF PLIA+ AND PLIA SCORE
Interobserver variability for the assessment of PLIA+ and PLIA score was analysed in 50 randomly selected cases by two independent observers (K. Kuroda and Y. Nagano). Repeated classification for 50 cases by one observer (K. Kuroda) was performed for intraobserver variability analysis three months after the first classification.
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSIS
Coronary angiograms, obtained at baseline and midterm follow-up, were analysed using a computer-based system with edge-detection techniques (QCA-CMS5.1; Medis Medical Imaging Systems, Leiden, the Netherlands). Late loss was defined as the difference between the minimum lumen diameter after the procedure and at midterm follow-up.
OUTCOME VARIABLES AND DEFINITIONS
Clinical outcome data (mean 42.6±22.1 months) were obtained by reviewing outpatient records or telephone interview for death, myocardial infarction (MI; defined according to the World Health Organization definition using creatine kinase and creatine kinase-MB increases), target lesion revascularisation (TLR), and a composite of death, MI, and TLR (major adverse cardiac events [MACE]). TLR was performed clinically. These data were obtained by personnel blinded to follow-up OCT findings. The primary endpoint of this study was TLR during the follow-up period. TLR required >1 year after PCI was defined as late-phase TLR6. All deaths were considered cardiac-related unless an unequivocal non-cardiac cause could be established.
STATISTICAL ANALYSIS
SPSS software, Version 23 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Qualitative data are presented with frequencies, and quantitative data are shown as mean±SD. For continuous variables, comparisons between two groups were performed using a two-tailed, unpaired t-test, or a Wilcoxon test. Discrete variables are presented as percentages, and comparisons were performed by chi-squared analysis or Fisher’s exact test. Between-group comparisons were performed with the one-way ANOVA test for continuous variables. Interobserver and intraobserver variabilities were quantified using kappa concordance analysis. Cox hazard regression analyses were performed to identify independent predictors of PLIA+ and TLR. Survival curves were constructed using Kaplan-Meier estimates and compared with the log-rank test. Hazard ratios and 95% confidence intervals were calculated by Cox proportional hazards regression model. Variables which showed a statistically significant difference in the univariate analyses were entered into a multivariate analysis using a backward stepwise selection to obtain the final model. At each step, the least significant variable was discarded from the model, until all variables in the model reached p<0.20; p<0.05 was considered statistically significant.
Results
PATIENT AND LESION CHARACTERISTICS
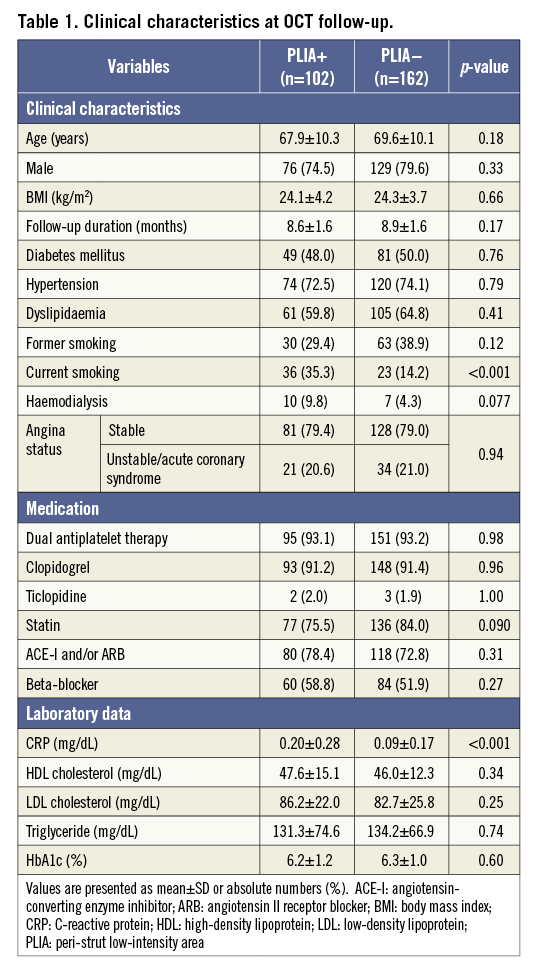
A total of 484 patients (1,038 lesions) were enrolled in the registry. Among them, 272 patients (324 lesions) who were treated with EES underwent follow-up OCT examination six to 12 months after PCI. After excluding eight patients, 264 patients (264 lesions) were ultimately included (Figure 3). PLIA was identified in 102 patients (PLIA+ group), while the remaining 162 patients did not exhibit PLIA (PLIA– group). The groups did not differ significantly with regard to medications at the follow-up OCT. The rates of current smoking and C-reactive protein (CRP) level were significantly higher in the PLIA+ group (Table 1). In the quantitative angiographic analysis, although pre- and post-intervention findings were comparable, the minimum lumen diameter at follow-up was significantly smaller, and follow-up percentage diameter stenosis and late loss were significantly greater in the PLIA+ group (Table 2).
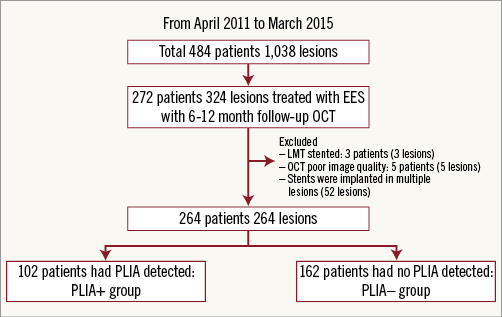
Figure 3. Study population.
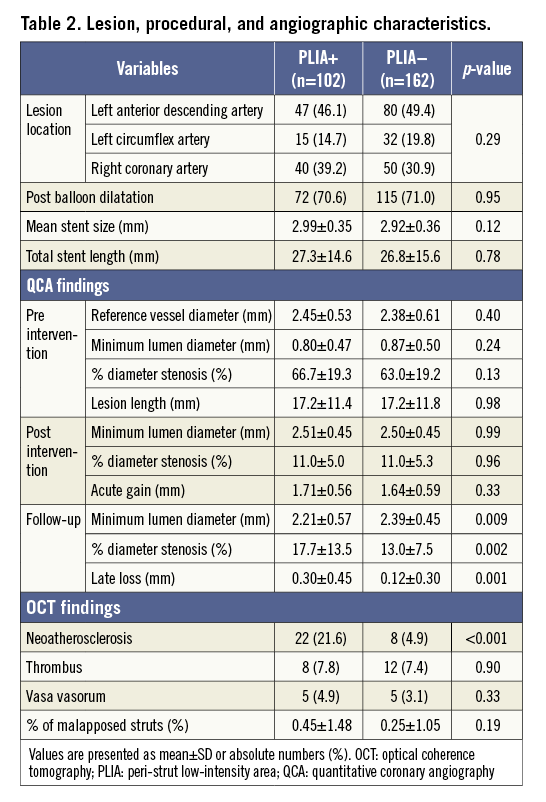
RISK FACTORS FOR PLIA
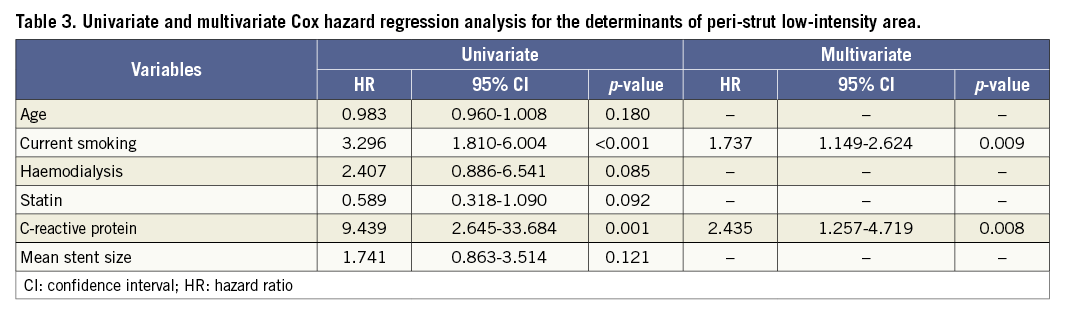
All patient and lesion characteristics were analysed by univariate and multivariate Cox hazard regression analyses to identify risk factors for PLIA+ (Table 3). Multivariate analysis showed that CRP level and current smoking were independently associated with PLIA+.
CLINICAL OUTCOMES
Overall, 22 patients experienced TLR (Table 4). All TLR events were angiographically documented in our hospital, and eight patients had fractional flow reserve measurement prior to TLR. In other cases, TLR was performed due to symptomatic chest pain or severe in-stent restenosis (>90%). Twelve patients required early-phase (<1 year) TLR and eight patients at the timing of follow-up OCT. Ten patients underwent late-phase (>1 year) TLR. Detailed QCA and OCT findings at midterm follow-up are shown in Supplementary Table 1.
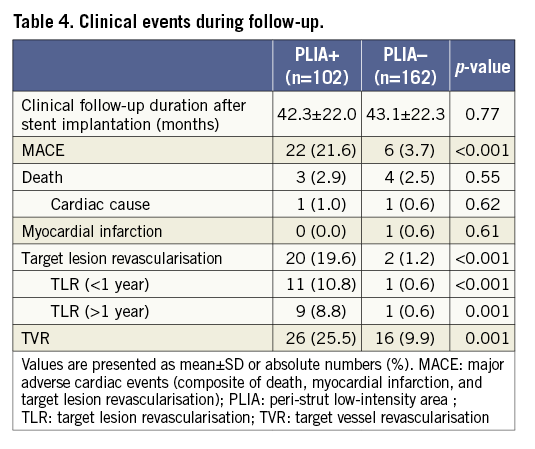
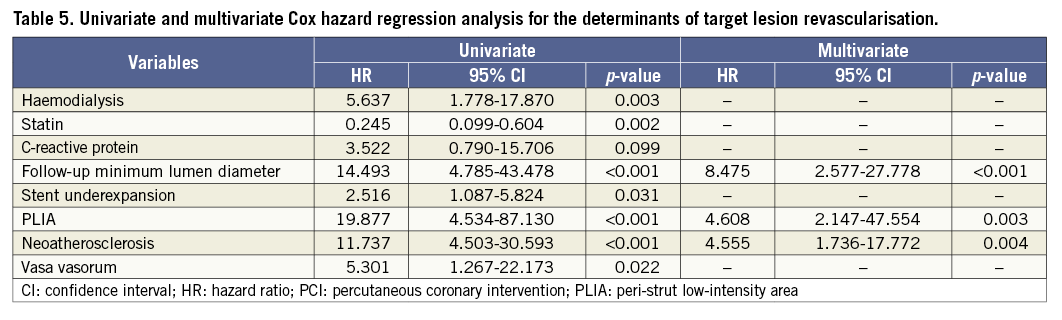
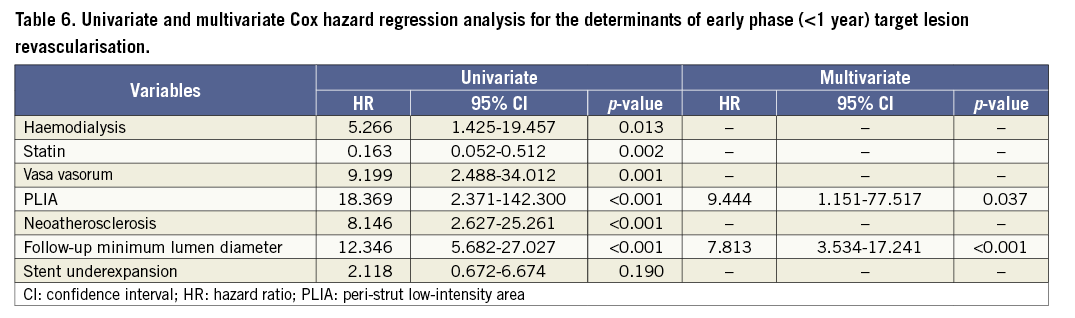
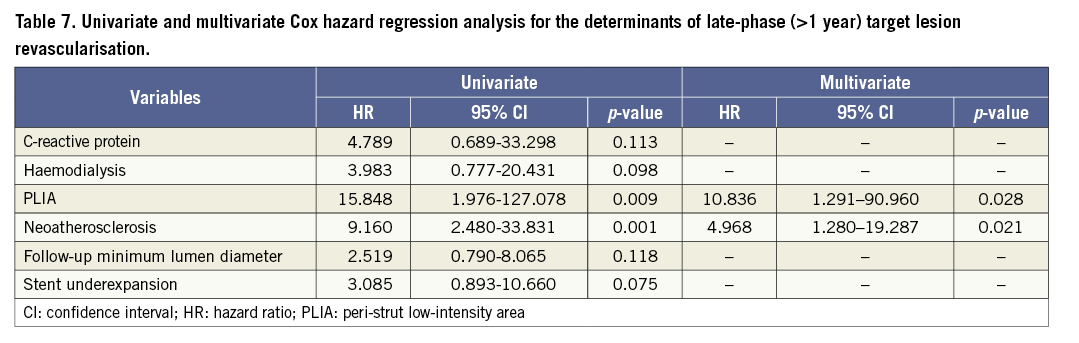
Patients with PLIA had a higher incidence of TLR during the clinical follow-up (Table 4, Figure 4A). Supplementary Figure 1 shows the number of patients who suffered from TLR annually during follow-up. Multivariate analysis indicated that PLIA+, neoatherosclerosis, and smaller follow-up minimum lumen diameter were independently associated with TLR (Table 5). TLR incidence at the early phase was significantly higher in the PLIA+ group (PLIA+: 10.8% vs. PLIA–: 0.6%, p<0.001) (Figure 4B). Additionally, in a landmark analysis one year after stent implantation, PLIA+ was associated with higher incidence of late-phase TLR after stenting (PLIA+: 11.7% vs. PLIA–: 0.7%, p<0.001) (Figure 4C). Multivariate analysis indicated that PLIA+ was independently associated with both early- and late-phase TLR (Table 6, Table 7).
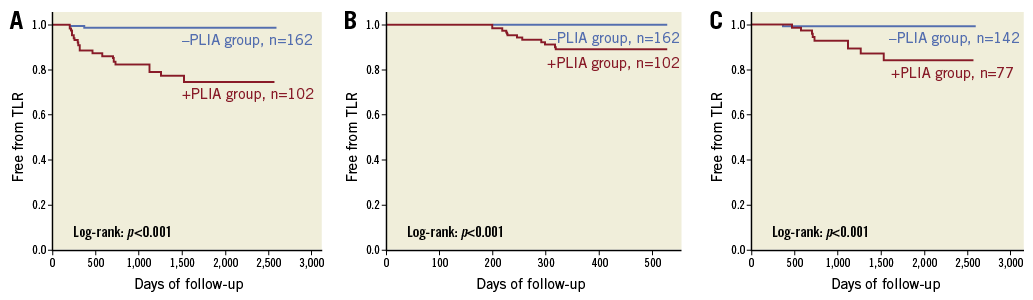
Figure 4. Survival analysis according to the presence or absence of peri-strut low-intensity area (PLIA). A) Kaplan-Meier survival curves of target lesion revascularisation (TLR) stratified according to the presence or absence of PLIA. B) Kaplan-Meier survival curves of early-phase (<1 year) TLR stratified according to the presence or absence of PLIA. C) Landmark analysis (>1 year) of TLR stratified according to the presence or absence of PLIA.
QUANTITATIVE AND QUALITATIVE ASSESSMENT OF PLIA
According to the quantitative assessment of PLIA, 63 patients were assigned PLIA score 1, 25 were assigned score 2, and 14 were assigned score 3. Patients with PLIA score 1 had significantly lower CRP levels than those with PLIA scores 2 and 3 (score 1: 0.12±0.22 vs. score 2: 0.28±0.26, p=0.042; score 1 vs. score 3: 0.45±0.38, p=0.030, respectively) (Figure 5). Patients with PLIA scores 2 and 3 had a significantly higher TLR incidence than patients with PLIA score 1 during the clinical follow-up (score 1: 3.2% vs. score 2: 40.0%, p<0.001; score 1 vs. score 3: 57.1%; p<0.001, respectively) (Figure 6A). Moreover, patients with PLIA scores 2 and 3 had a significantly higher incidence of early- and late-phase TLR than patients with PLIA score 1 (Figure 6B, Figure 6C). CRP level and TLR rate did not significantly differ between the PLIA– group and patients with PLIA score 1. Additionally, the prevalence of +PLIA struts was significantly higher in the TLR+ group, not only in the early-phase TLR but also in the late-phase TLR (Supplementary Figure 2).
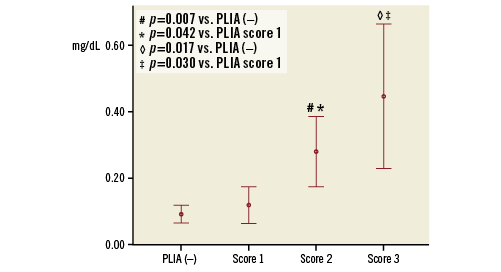
Figure 5. Association between peri-strut low-intensity area score and C-reactive protein level.
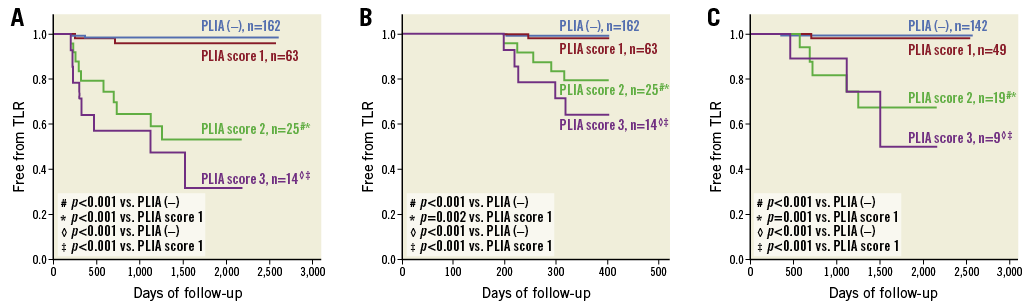
Figure 6. Survival analysis according to peri-strut low-intensity area (PLIA) score. A) Kaplan-Meier survival curves of target lesion revascularisation (TLR) stratified according to PLIA score. B) Kaplan-Meier survival curves of early-phase (<1 year) TLR stratified according to PLIA score. C) Landmark analysis (>1 year) of TLR stratified according to PLIA score.
REPRODUCIBILITY OF PLIA+ AND PLIA SCORE
The interobserver and intraobserver variabilities for PLIA detection were within the acceptable range (interobserver, kappa=0.920; intraobserver, kappa=0.960). Additionally, the interobserver and intraobserver variabilities for the classification of PLIA score were within the acceptable range (interobserver, kappa=0.877; intraobserver, kappa=0.908).
Discussion
CLINICAL EFFECT OF PLIA
Several studies have shown a potential association between PLIA and neointimal proliferation. In a previous study, we found that the average neointimal thickness was significantly greater on +PLIA struts than on –PLIA struts2. Additionally, Sato et al reported that the presence and extent of PLIA appeared to be significantly associated with neointimal area 12 months after everolimus-eluting bioresorbable scaffold implantation7. These findings were confirmed by a more recent investigation of the relationship between PLIA and late healing characteristics after stenting. Choi et al reported significant correlations between the severity of PLIA and degree of neointima as well as angiographic late loss in patients treated with bare metal stents and first- and second-generation DES8. These studies, however, did not assess the potential relationship of PLIA with clinical events because of the limited number of patients and follow-up duration. In this study, we successfully clarified a significant relationship between PLIA and TLR by enrolling a total of 264 patients with a median follow-up of 42.6 months after EES implantation. Interestingly, PLIA was associated with both early- and late-phase TLR. Notably, this association was independently observed from luminal narrowing during midterm OCT investigation, which can be one of the strongest predictors of TLR. Therefore, we believe that PLIA provides incremental prognostic information in addition to a simple evaluation of luminal narrowing. Although the detailed mechanisms of this relationship are still speculative, fibrin deposition, extracellular matrix accumulation, continuous inflammation, or formation of neoatherosclerosis might contribute8.
Conversely, patients without PLIA on midterm OCT findings experienced almost no late-phase TLR during follow-up. Therefore, the absence of PLIA on midterm OCT images might suggest a low inflammatory reaction to the implanted stent, possibly indicating its long-term patency. Additionally, patients with PLIA score 1 had a statistically equivocal TLR rate compared to those without PLIA. Since PLIA is a relatively common finding after stenting, not only its presence but also the qualitative assessment of its severity can be clinically important for the risk stratification of patients treated with coronary stenting.
In our study, the TLR rate until one year was 4.5%, and the rate of late-phase TLR was 3.8% with a mean clinical follow-up of 42.6 months. In a previous study, the ischaemia-driven TLR rate until one year in EES-implanted patients was 3.5% and the rate of late-phase TLR was 5.1% in a five-year follow-up9. Considering that the mean lesion length was 2.5 mm longer (17.2 mm and 14.7 mm, respectively) and the reference vessel diameter was 0.36 mm smaller (2.41 mm and 2.77 mm, respectively) than the previous study with haemodialysis patients (6.4%), the early and late TLR rates in the present study can be considered reasonable.
RISK FACTORS FOR PLIA
We found that increased CRP level was independently associated with PLIA+. In addition, patients with PLIA score 1 had significantly lower CRP levels than those with PLIA scores 2 and 3. Increased CRP level has been reported to reflect not only systemic but also local vascular inflammation in patients with CAD10. Therefore, we speculate that PLIA on midterm OCT images might represent ongoing inflammation surrounding the stent struts. Several histological studies have shown that inflammatory reactions play important roles in the initiation and proliferation of neointimal hyperplasia and restenosis after stent implantation11. Therefore, considering both fibrin deposition and excessive extracellular matrix remodelling, PLIA might reflect sustained localised inflammation with ongoing biological processes, potentially leading to early- and late-phase TLR.
Our study showed that neoatherosclerosis was more frequently observed in lesions with PLIA than in those without. We previously reported that neoatherosclerosis has been associated with future TLR and high CRP levels4. PLIA is mainly detected on midterm OCT images, whereas neoatherosclerosis is mainly detected on long-term OCT12. Considering that both neoatherosclerosis and PLIA have significant relationships with increased CRP levels, these findings might be linked to each other through inflammatory reaction after stenting.
Current smoking was an independent risk factor for PLIA+. Gao et al reported that current smoking was associated with heterogeneous neointima on six- to 12-month follow-up OCT images after sirolimus-eluting stent implantation13. Heterogeneous neointima has been considered another sign of abnormal vascular healing that can correspond to abnormal tissues, including a proteoglycan-rich myxomatous matrix14. Moreover, Mandraffino et al reported that current smokers showed a significantly enhanced biglycan-mRNA expression compared with control participants15. Biglycan is a ubiquitous component of the extracellular matrix that orchestrates several physiological functions and is considered an early initiator of arterial atherosclerosis. Although these findings, including ours, cannot show a causal relationship of current smoking and PLIA, they might represent a link between current smoking and the development of abnormal vascular healing represented by PLIA.
Limitations
This study has several limitations. First, it was a single-centre, non-randomised, retrospective study including only patients with follow-up OCT examination six to 12 months after stenting, indicating a potential patient selection bias. Second, detailed mechanisms of the PLIA phenomenon are still unclear, partly due to a lack of histological validation. Third, vascular healing response depends on initial plaque morphology. However, pre-PCI OCT was available only for some patients (116 of 264 patients). Stent edge restenosis was observed in nine patients, and pre-PCI OCT was available in four patients. Among these four patients, edge lipid plaque was observed in two patients. Edge plaque morphology may be connected with future TLR, as already known, but it cannot be stated clearly due to the limited number of pre-PCI OCT examinations. A further study with pre-PCI OCT is required. Fourth, stent underexpansion was an important risk factor for future TLR. However, stent underexpansion did not remain an independent risk factor for TLR in the multivariate Cox hazard regression analysis. In our study, 47 patients were implanted with overlapping stents, and 61.7% of such patients were consequently classified with stent underexpansion by definition. In overlapping stent cases, because the culprit lesion was long, large differences between the distal (mean 5.0 mm2) and proximal reference lumen areas (mean 7.2 mm2) existed. Therefore, in such cases, stents were more likely to be classified as underexpanded, even in cases with optimal expansion, which might be the reason why it does not remain as a risk factor for TLR. Finally, the natural time course of PLIA remains unclear. A serial study that follows changes in PLIA is warranted to clarify the progression of PLIA.
Conclusions
In this study, high CRP level and current smoking were independently associated with PLIA+. Patients with PLIA had a significantly higher TLR incidence during the follow-up period. Considering the association between CRP level and the severity of PLIA, PLIA might reflect sustained localised inflammation, potentially leading to early- and late-phase TLR. Detailed stent assessment by midterm follow-up OCT may help to predict future stent failure in patients with CAD.
| Impact on daily practice This study demonstrated that patients with PLIA on midterm follow-up OCT imaging had a significantly higher incidence of early- and late-phase TLR than patients without PLIA, suggesting that detailed stent assessment by midterm follow-up OCT may help to predict future stent failure in patients with CAD. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. The number of patients who suffered from TLR annually during follow-up.
Supplementary Figure 2. The association between the prevalence of PLIA and TLR in patients with PLIA.
Supplementary Table 1. QCA and OCT findings at follow-up OCT in patients with late-phase TLR.
To read the full content of this article, please download the PDF.

