
Abstract
Aims: We sought to investigate subclinical neoatherosclerotic changes and the healing response to sirolimus-eluting stents (SES) to clarify the clinical safety and the neointimal pathology of SES more than 10 years after implantation.
Methods and results: We investigated a total of 180 SES without stent failure in 103 patients who underwent optical coherence tomography (OCT) examination of stented vessels more than five years after implantation. We assessed the presence or absence of neoatherosclerosis and the healing process using OCT and compared the results between stents at five to 10 years after implantation (Group A, 114 stents with 19,873 struts) and stents more than 10 years after implantation (Group B, 66 stents with 10,937 struts). The median stent age of the whole cohort was 9.4 (7.8–10.9) years. In the OCT analysis, Group B was associated with higher frequencies of neoatherosclerosis than Group A. However, the prevalence of uncovered stents and stent malapposition was not significantly different between the two groups.
Conclusions: SES of more than 10 years of age are associated with a higher frequency of OCT-defined neoatherosclerosis than SES of five to 10 years of age, indicating continuous development of neoatherosclerosis beyond 10 years after implantation.
Abbreviations
DES: drug-eluting stent(s)
IQR: interquartile range
OCT: optical coherence tomography
SES: sirolimus-eluting stent(s)
Introduction
Sirolimus-eluting stents (SES) were introduced in the early 2000s and rapidly emerged in clinical practice, showing better restenosis rates within one year than those of bare metal stents1,2. Even in the era of second-generation drug-eluting stents (DES), millions of patients who have been treated with first-generation DES are still alive, and these patients will naturally become older as time passes. Therefore, longer follow-up data will be valuable in the future. However, some studies have raised concerns regarding the constant increase in stent failure after one year, which has been attributed to delayed healing3,4 and neoatherosclerosis by pathological investigations5,6. The attenuation of the annual risk of adverse events from five to 10 years is still controversial7,8, and the clinical safety and underlying pathophysiology of SES at more than 10 years after stenting are unknown. Optical coherence tomography (OCT) is a high-resolution imaging modality that enables the in vivo assessment of in-stent characteristics9. Similar to pathological investigations, stent malapposition, uncovered struts, and ruptured neoatherosclerosis detected by OCT are thought to be the major leading causes of very late stent thrombosis10-12. We sought to investigate the neointimal abnormality of SES with an age of more than five years.
Methods
STUDY POPULATION
The institutional database of cardiac catheterisation at Tsuchiura Kyodo General Hospital between October 2012 and September 2017 (which included data from 2,359 OCT examinations in 1,392 patients) was searched to identify patients in whom OCT examination was performed in segments with previously implanted SES exhibiting no stent failure. According to the protocol of angiographic and OCT follow-up, coronary angiography was scheduled as the clinical practice at the physician’s discretion for patients who had received coronary stents. In addition to coronary angiography, OCT examinations were performed of vessels in which stents had been implanted more than three years previously in patients from whom informed consent was obtained. From the database, we selected patients for analysis if OCT provided sufficient image quality of the neointimal morphology of SES at more than five years after implantation. A total of 279 SES from 127 patients were identified for the analysis. All SES were implanted between June 2004 and August 2010. We excluded stents implanted within a stent (n=91), in-stent restenosis requiring revascularisation (n=6), and stents with insufficient image quality (n=2) from the analyses. No stent with an ongoing thrombotic event was included in the final data set. No stent thrombotic event occurred in this population. Therefore, the final data set included 180 SES from 103 patients. In this population, 19 stents were subjected to OCT examination as the non-target lesion at the time of PCI for the other culprit lesion. The remaining 161 stents were subjected to OCT examination in accordance with the institutional protocol, as described previously. Data on baseline patient characteristics were collected by reviewing medical charts. Angiographic data and OCT findings were compared between stents of five to 10 years of age (Group A, 114 stents) and stents of more than 10 years of age (Group B, 66 stents), according to previous studies investigating long-term outcomes at five and 10 years. The study protocol was approved by the institutional review board and, prior to catheterisation, all patients provided written informed consent for institutional database registration for future clinical research.
ANGIOGRAPHIC ANALYSIS, OCT IMAGE ACQUISITION AND ANALYSIS
Quantitative coronary angiography was performed using offline software (QCA-CMS; Medis medical imaging systems, Leiden, the Netherlands). The minimum lumen diameter and reference diameter were measured in diastolic frames from orthogonal projections.
OCT images were acquired after diagnostic coronary angiography or percutaneous coronary intervention procedure for non-target lesions using frequency-domain OCT systems (ILUMIEN™; St. Jude Medical, St. Paul, MN, USA, or Lunawave®; Terumo, Tokyo, Japan). The technique of OCT image acquisition has been described elsewhere9. A 2.7 Fr OCT imaging catheter (Dragonfly™ JP; LightLab Imaging [now St. Jude Medical], or FastView®; Terumo, Tokyo, Japan) was advanced distal to the stent, and contrast medium was injected at a flush rate of 3.0 to 4.0 ml/s through the guiding catheter; pullback was initiated as soon as the blood was cleared. Cross-sectional OCT images were analysed at intervals of 1.0 mm for evaluation. The minimal stent and lumen area were semi-automatically traced and determined by using proprietary software (St. Jude Medical or Terumo) at the Tsuchiura Kyodo Hospital OCT Laboratory, based on expert consensus documents9. Qualitative OCT assessment was performed by two experienced investigators (T. Yonetsu and M. Hoshino). Discordance between the two investigators was resolved through consensus reading. Lipids were defined as diffusely bordered signal-poor regions with rapid signal attenuation (Figure 1A). A lipid-laden neointima was defined as a neointima with lipids exceeding 90 degrees in circumference and 0.5 mm in length. A thin-cap fibroatheroma-like neointima was defined as a lipid-laden neointima with a fibrous cap thickness <65 µm in the thinnest portion and a lipid angle >180 degrees (Figure 1B)9. Calcification was defined as a clearly delineated signal-poor region with low backscatter (Figure 1C). The stent was considered to exhibit neoatherosclerosis when a lipid-laden neointima or calcification was present. Intracoronary thrombi were defined as signal-rich, low-backscattering protrusions or high-backscattering protrusions within the lumen showing signal-free shadowing in OCT images (dimension ≥250 μm) (Figure 1D). Neointimal rupture was defined as a discontinuity of the fibrous cap overlying a lipid-laden neointima (Figure 1E). Coronary evagination was defined as the presence of an outward bulge in the luminal vessel contour between apposed struts where the maximum depth of the bulge exceeded the actual strut thickness, as measured semi-automatically from the deepest point in the bulge to the stent area trace (Figure 1F)13,14. Strut-based analysis was performed at 1 mm intervals within the stented lesion by an experienced investigator (E. Usui), blinded to stent age or the patient’s clinical characteristics. Cross-sections with side branches or overlapping segments were excluded from the analysis. We measured the distance between the centre of the blooming artefact and the luminal surface and, when the distance was >167 μm (metal thickness [140 μm] + abluminal polymer thickness [7 μm] + OCT axial resolution [20 μm]), the strut was considered malapposed15. An apposed strut was classified as embedded if the distance between the strut and luminal surface was equal to or less than 83 μm and as protruding if the distance was >83 μm and ≤167 μm15. All visible struts in each 1 mm interval cross-section were classified into five categories based on their coverage and apposition, as follows: 1) covered and embedded; 2) covered and protruding; 3) covered and malapposed; 4) uncovered and apposed; and 5) uncovered and malapposed. Representative OCT images are shown in Figure 2. A stent was defined as uncovered/malapposed if at least one cross-section showed a ratio of uncovered/malapposed struts to total struts of >0.3.
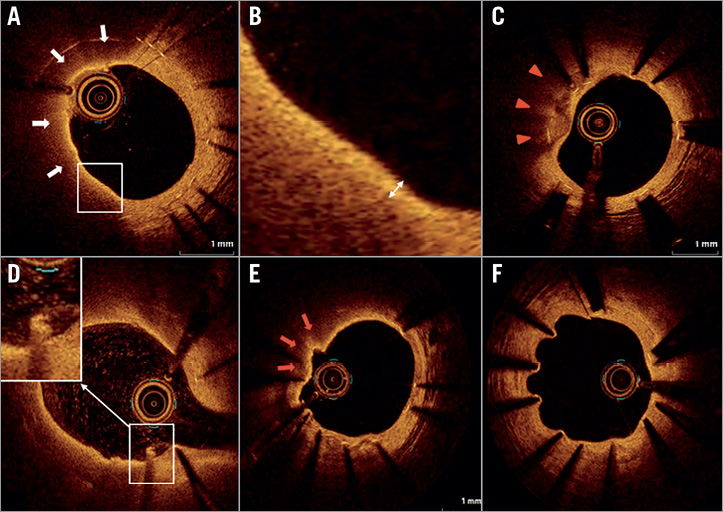
Figure 1. Representative images of OCT findings. A) Lipid-laden neointima (white arrows). B) A thin-cap fibroatheroma-like neointima. Cap thickness was measured at the thinnest part (double-headed arrow). C) Calcified neointima (red arrowheads). D) Intracoronary thrombus. E) Neointimal rupture (red arrows). F) Coronary evagination.
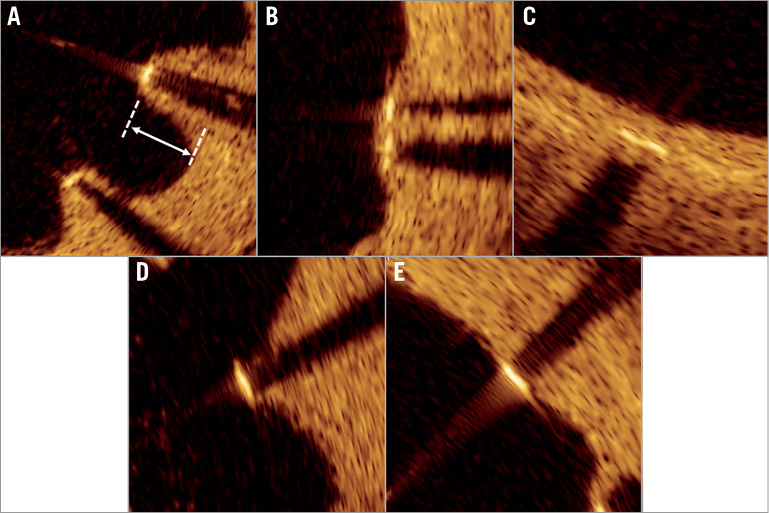
Figure 2. Representative images of strut apposition and neointimal coverage of a sirolimus-eluting stent. A) Covered and malapposed. B) Covered and protruding. C) Covered and embedded. D) Uncovered and malapposed. E) Uncovered and apposed.
STATISTICAL ANALYSIS
Data were analysed on a per-patient, per-stent, per-cross-section, or per-strut basis. Categorical data were expressed as numbers and percentages and compared via the χ2 test or Fisher’s exact test, as appropriate. Continuous variables were expressed as the mean±standard deviation for normally distributed variables or as the median (25th-75th percentile) for non-normally distributed variables and compared using Student’s t-tests and Mann-Whitney U tests, respectively. Interobserver variability for lipid-laden and calcified neoatherosclerosis was assessed by two independent observers. Intraobserver variability was assessed by re-analysis of data from a single observer. Univariable and multivariable logistic regression analyses were performed to determine the predictors of neoatherosclerosis. The variables that were found to be significant in the univariable analyses (p<0.05) were included all together in the multivariable model. A generalised estimating equation approach was exercised to consider within-subject correlations resulting from the analysis of multiple stents within a single patient. For the cross-section-level and strut-level analyses, we estimated proportions in each group and their odds ratios by using a generalised linear mixed model to account for a multilevel data structure. We included stent age (five to 10 years vs. >10 years) as a fixed effect and patients, stents, and cross-sections (for strut-level analysis) or patients and stents (for cross-section-based analysis) as random intercepts. SPSS, Version 23.0 (IBM Corp, Armonk, NY, USA) and R statistics version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for the statistical analyses. A p-value <0.05 was considered statistically significant.
Results
PATIENT CHARACTERISTICS AND ANGIOGRAPHIC DATA
The baseline patient characteristics are summarised in Table 1. The median stent age was 8.3 (interquartile range [IQR] 7.4–9.3) years in Group A, 11.3 (IQR 10.8–12.2) years in Group B, and 9.4 (IQR 7.8–10.9) years in the whole cohort. There were no significant differences in baseline clinical characteristics between the two groups except for angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use. The frequency of stents implanted at culprit lesions due to acute coronary syndrome at the index procedure was 14/114 (12.3%) in Group A and 15/66 (22.7%) in Group B (p=0.07). Neither mean stent size nor length differed between the two groups. Angiographic minimal lumen diameter, reference diameter, and stenosis diameter were not significantly different between the two groups (Table 2).
OCT ANALYSIS
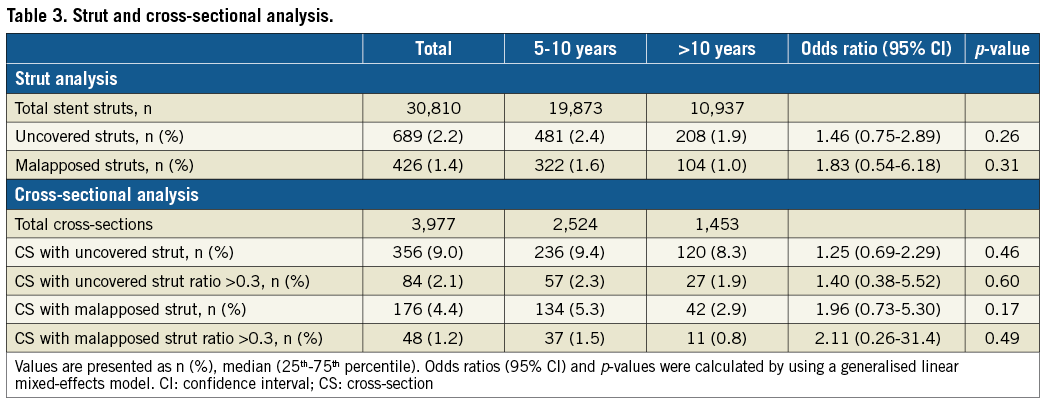
There was good interobserver and intraobserver concordance in the diagnosis of lipid-laden (κ=0.91, 0.93) and calcified (κ=0.89, 0.91) neoatherosclerosis. OCT findings were compared between Group A (114 stents with 19,873 struts) and Group B (66 stents with 10,937 struts). In OCT analysis, Group B was associated with higher frequencies of lipid-laden neoatherosclerosis and calcified neoatherosclerosis and a lower frequency of evagination than Group A. However, the prevalence of uncovered stents and stent malapposition was not significantly different between the two groups (Table 2, Figure 3). The same tendencies were observed in the strut-level and cross-section-level analyses (Table 3). The prevalence of intracoronary thrombi, neointimal rupture, and thin-cap fibroatheroma-like neointimas showed no significant differences between the two groups. The follow-up OCT findings showed no significant differences between the two groups divided by the baseline clinical presentation, specifically, patients with acute coronary syndrome and those with stable angina pectoris (Supplementary Table 1). Univariable logistic regression analysis showed that the absence of β-blocker use and a stent age of more than 10 years were associated with the presence of neoatherosclerosis. In the multivariable logistic regression analysis, a stent age of more than 10 years was the only independent predictor of neoatherosclerosis (Supplementary Table 2).
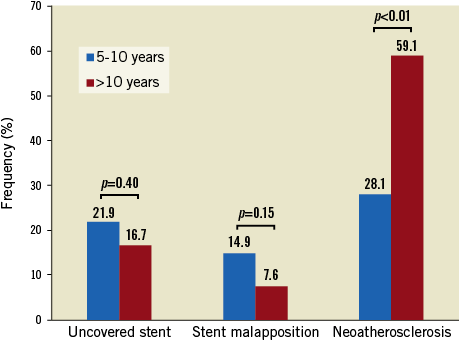
Figure 3. Comparison of neointimal characteristics in stents of age 5-10 years versus >10 years.
Discussion
To the best of our knowledge, this is the first study to investigate the prevalence of subclinical complications including malapposition, uncovered strut, and neoatherosclerosis, in SES with a stent age of more than 10 years and stents less than 10 years of age. The main findings of our study are as follows. SES of more than 10 years of age are: 1) significantly more frequently associated with neoatherosclerosis, and 2) less frequently, albeit non-significantly, associated with strut uncoverage and malapposition than are SES of five to 10 years of age.
In a 10-year observational study, Galløe et al showed that major adverse cardiac events occurred in 32.5% of patients who underwent SES implantation, with a steady annual rate of 2.6% after the first year. The authors also revealed that stent thrombosis occurred in 13.3% of patients with SES, with a steady annual rate of 1.2% after the first year8. In contrast, in a long-term follow-up trial, Yamaji et al revealed that the annual risk of ischaemia-driven target lesion revascularisation and definite stent thrombosis was significantly higher between one and five years than between five and 10 years after first-generation DES implantation, which suggested attenuation of the annual risk of late stent complications over time7. Thus, although it remains controversial whether the annual risk of stent thrombosis increases or decreases, patients who receive SES implantation continue to suffer from stent failure in their chronic phase. Recently, Adriaenssens et al reported that the predominant OCT findings associated with very late stent thrombosis occurring at a median of five years after stenting were neoatherosclerosis and uncovered struts for all kinds of stents, while for first-generation DES it was uncovered struts12. Several OCT studies based on retrospective data have also indicated that malapposition, neoatherosclerosis and uncovered struts are the major indicators of very late stent thrombosis10,11.
Conversely, the prognostic impact of neoatherosclerosis has also been reported, although the sample size involved and prospective studies are limited. Kuroda et al retrospectively studied the association between neoatherosclerosis and future events and revealed that, after a follow-up duration of 50.9±7.7 months, the presence of neoatherosclerosis was an independent predictor of major adverse cardiac events16. Ueda et al performed a single-centre prospective study using angioscopy and reported that, after a follow-up interval of 4.3±2.4 years, in-stent yellow neointima at one year after DES implantation and the absence of statin therapy were risk factors for very late stent failure-associated events17. These data suggest that neoatherosclerosis may serve as a surrogate marker of the risk for future adverse cardiac events.
In the present cross-sectional observational study, we investigated the frequency of subclinical neointimal abnormality of SES with a stent age of more than five years. Our main findings may explain why patients continuously suffer from a risk of very late stent failure after SES implantation and indicate that this risk may develop over 10 years after implantation, indicating that the neointima of SES proliferates continuously beyond five and 10 years. We can also speculate that the dominant mechanism of stent thrombosis may shift from delayed neointimal healing to neoatherosclerosis. Furthermore, if the main mechanism of stent thrombosis shifts, the therapeutic strategy for secondary prevention should also be shifted, from an intensive antiplatelet therapy for uncovered or malapposed struts to intensive lipid-lowering therapy and glycaemic control to prevent neoatherosclerotic changes within the stent. Among the six patients with stent failure during the study period, all six stents developed neoatherosclerosis. One of these stents showed disruption of the lipid-laden neointima. No thrombi, uncovered struts, malapposed struts, or evaginations were observed in these six SES. Furthermore, three patients did not receive statin treatment, and two patients had poor glycaemic control (HbA1c >7.0%). Since the present study was based on cross-sectional, retrospective analyses, it remains elusive how often neoatherosclerosis resulted in clinical events. Moreover, the difference in clinical impact between lipid-laden neoatherosclerosis and calcified neoatherosclerosis has not been elucidated. However, the fact that all six stents showing stent failure exhibited neoatherosclerosis might in part suggest the contribution of neoatherosclerosis to stent failure in a very late phase, rather than delayed neointimal healing. Further studies are needed to clarify whether there is any difference in culprit lesion neoatherosclerosis of stent failure and the long-standing silent neoatherosclerosis observed in the present study.
Study limitations
This study has several limitations that should be considered when interpreting the results. First, this was a retrospective, observational study that included subjects from a single centre. Although OCT examination was scheduled and performed in accordance with the institutional protocol, the sample size of our data set (n=279) accounted for only a small proportion of the total number of SES implanted throughout the period (n=1,567). Selection bias and the limitation of the registry design may have affected the results. Second, since the prognostic impact of neoatherosclerosis has not been fully investigated in a large-scale prospective study, our results considering neoatherosclerosis as one of the surrogate markers of future events are hypothesis-generating and should be carefully interpreted. Third, we excluded stent-in-stent lesions and overlapping segments, both of which can affect the formation of neoatherosclerosis. Therefore, the analysis does not completely reflect the real-world situation of long-term follow-up after SES implantation. Fourth, since the number of SES within five years of age in our institutional database was very small (n=6), we limited the investigation of the time course of in-stent characteristics to SES at more than five years after implantation. Finally, OCT images at the index procedure were not available. Therefore, the impact of baseline plaque characteristics on the subsequent neointimal change could not be assessed. Laboratory data and medication status at the time of the index procedure and during the study period were also incomplete. These deficits may have caused an unfair interpretation of the long-term effects of clinical factors on neointimal morphology.
Conclusions
SES of more than 10 years of age are associated with a higher frequency of OCT-defined neoatherosclerosis than SES of five to 10 years of age, while uncovered/malapposed stents tend to be less frequent, albeit non-significantly, beyond 10 years after implantation than after five to 10 years of stenting. These results might be indicative of the continuous development of neoatherosclerosis and an increased risk of stent failure more than 10 years after SES implantation.
| Impact on daily practice SES of more than 10 years of age were associated with a higher frequency of OCT-defined neoatherosclerosis than SES of five to 10 years of age, while the prevalence of uncovered stents and malapposed stents tended to be lower in SES of more than 10 years of age than in the other group. These results might indicate that an increased risk of super-delayed SES failure might be attributable to continuous development of neoatherosclerosis, rather than delayed neointimal healing. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Association between baseline clinical presentation and follow-up optical coherence tomography findings.
Supplementary Table 2. Univariable and multivariable logistic regression analysis.
To read the full content of this article, please download the PDF.

