
Coronary stents are widely used for the treatment of patients with coronary artery disease. Drug-eluting stents (DES) have reduced the rate of late restenosis compared with bare metal stents (BMS). However, late and very late stent thrombosis have become a major concern in DES. Pathological findings from patients who died of very late DES thrombosis have demonstrated that incomplete re-endothelialisation, late acquired stent malapposition, and atherosclerosis within neointima (known as neoatherosclerosis) are important underlying substrates of very late DES thrombosis1. Rupture of neoatherosclerosis is the most common mechanism of very late DES thrombosis. Neoatherosclerosis in DES occurs earlier compared with BMS and develops continuously.
Present study
In the current issue of EuroIntervention, Usui et al report the prevalence of inadequate vascular responses in sirolimus-eluting stents more than 10 years after implantation by using optical coherence tomography (OCT)2.
The authors demonstrated that the prevalence of neoatherosclerosis was significantly higher in sirolimus-eluting stents more than 10 years after implantation compared with sirolimus-eluting stents five to 10 years after implantation, though the prevalence of uncovered and/or malapposed stents tended to be lower in sirolimus-eluting stents more than 10 years after implantation2. These results suggest a potentially increased risk of sirolimus-eluting stent failure due to continuous development of neoatherosclerosis beyond 10 years after implantation.
Neoatherosclerosis in DES
Pathohistologically, neoatherosclerosis is characterised by the accumulation of lipid-rich foamy macrophages within the neointima of stented coronary segments. Unlike native coronary disease, necrotic core formation in neoatherosclerosis occurs from macrophage apoptosis in the absence of pathologic intimal thickening and lipid pools. The neoatherosclerosis in DES is observed at six months after implantation, though that in BMS is seen at two years after implantation1. There are two possible mechanisms responsible for the development of neoatherosclerosis in DES. One mechanism is incomplete re-endothelialisation induced by the drug from DES. Poor cell-to-cell junctions lead to impairment of endothelial barrier function, which allows lipoproteins and macrophages to infiltrate the neointima. The other mechanism is chronic inflammation promoted by the polymer of DES. Accumulation of macrophages, lymphocytes, and giant cells around the DES struts contribute to the development of neoatherosclerosis. These mechanisms responsible for the development of neoatherosclerosis have been confirmed by pathological studies of DES months to years after implantation. However, there has been no pathological study investigating neoatherosclerosis beyond a decade after DES implantation. It remains unclear whether incomplete re-endothelialisation and chronic inflammation have a lasting influence on the neoatherosclerosis in the extremely late phase after DES implantation.
Stent architecture (e.g., stent strut thickness) and components (e.g., metal, polymer, and drug) influence the development of neoatherosclerosis. The first-generation DES had the disadvantages of thick metallic struts (140 μm for the CYPHER® sirolimus-eluting stent [Cordis, Johnson & Johnson, Miami, FL, USA], and 132 μm for the TAXUS™ paclitaxel-eluting stent [Boston Scientific Corp., Marlborough, MA, USA]) and poorly biocompatible permanent polymers. The second-/third-generation DES are equipped with thinner (<100 μm) cobalt-chromium or platinum-chromium stent struts, more biocompatible permanent polymers or biodegradable polymers, and lower drug loads with predominant use of limus agents. These features of second-/third-generation DES reduce vascular inflammation and improve the re-endothelialisation of stents. Nevertheless, recent pathological studies showed that the prevalence of neoatherosclerosis was not different between the first-generation DES and the second-/third-generation DES1. Therefore, neoatherosclerosis remains a major drawback even in the second-/third-generation DES.
Intravascular imaging allows us to examine neointimal tissue characteristics and detect in-stent neoatherosclerosis in living patients (Figure 1). An angioscopy study showed that yellow neointima suggestive of lipid-rich tissue or necrotic core was detected in 95% of DES at 10 months after implantation, though it was observed in 58% of BMS at four years after implantation3. A virtual histology intravascular ultrasound study demonstrated that necrotic core and dense calcium within neointima of DES increased with time (mean follow-up time: 11±8 months, range 4 to 33)4. A near-infrared spectroscopy study revealed that this technology was capable of identifying lipid-rich neointima in early (median follow-up time: 7 months, interquartile range 3 to 10) DES restenosis5. An OCT study disclosed that thin-cap fibroatheroma, which is recognised as a precursor lesion of plaque rupture, developed in the neointima of DES at 20 months after implantation6. These intravascular imaging studies consistently suggest that DES accelerate neoatherosclerosis.
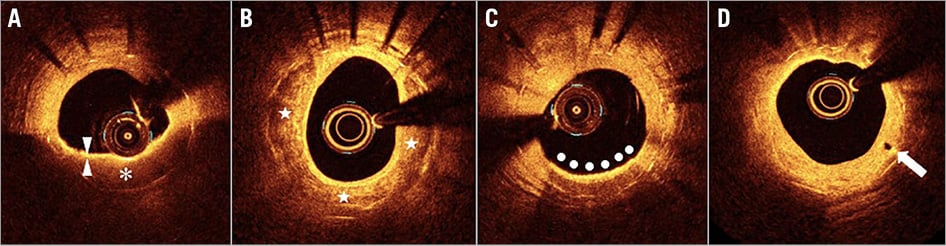
Figure 1. Optical coherence tomography findings of neoatherosclerosis. A) Lipid (asterisk) is characterised by a signal-poor region with a poorly delineated border. Fibrous cap (arrowheads) is characterised by a signal-rich tissue layer overlying a signal-poor region. B) Calcium (stars) is characterised by a signal-poor and heterogeneous region with a sharply delineated border. C) Accumulations of macrophages (bullets) are seen as confluent or punctate signal-rich regions that shadow underlying tissue. D) Neovascularisation (arrow) appears as a signal-poor void that is sharply delineated.
Neoatherosclerosis identified by intravascular imaging is associated with very late stent thrombosis. A retrospective OCT study showed that rupture of neointima with advanced neoatherosclerosis was seen in 63% of very late DES thrombosis7. Furthermore, a prospective angioscopy study named DESNOTE (Detect the Event of very late Stent failure from the drug-eluting stent NOT well covered by nEointima determined by angioscopy) demonstrated that yellow neointima identified at one year after DES implantation was an independent predictor of very late (mean follow-up time: 4±2 years) stent thrombosis8. Thus, the assessment of neoatherosclerosis by using intravascular imaging might be helpful for risk stratification of subsequent very late DES thrombosis.
Final remarks
In addition to the drug and polymer of DES, traditional clinical risk factors for native atherosclerosis influence the development of neoatherosclerosis in a very late phase after DES implantation. Previous OCT studies showed that current smoking, chronic kidney disease, lack of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockade use, and absence of statin therapy were associated with neoatherosclerosis in a very late phase after DES implantation9. According to the results of the study by Usui et al2, comprehensive risk factor control and optimal medical therapy seem to be necessary for the long period of beyond a decade to prevent very late stent failure in patients treated with DES.
Conflict of interest statement
T. Kubo has received lecture fees from Abbott Vascular Japan. T. Akasaka has received lecture fees and research funds from Abbott Vascular Japan.

