Abstract
Aims: To use optical coherence tomography (OCT) to evaluate the time course, risk factors, and clinical implication of in-stent neoatherosclerosis.
Methods and results: The neointimal characteristics of 152 lesions, 128 drug-eluting stents (DESs) and 24 bare metal stents (BMSs), with >50% percent cross-sectional area (CSA) neointimal stenosis were evaluated. Neoatherosclerosis was defined as neointima with presence of lipid or calcification. Neoatherosclerosis was observed in 54 lesions (35.5%, 35 DESs and 19 BMSs). Median time to follow-up was 70.7 months in lesions with neoatherosclerosis (longer than lesions without neoatherosclerosis [13.4 months, p<0.001]): 58.7 months in DES-treated lesions and 129.5 months in BMS-treated lesions (p<0.001). The optimal cut-off time to predict neoatherosclerosis in DES-treated lesions was 30 months with a sensitivity of 91.4% and a specificity of 72.0% (area under curve: 0.839, 95% confidence interval: 0.764-0.898, p<0.001). Independent risk factors for neoatherosclerosis were stent age, use of first-generation DES and hypertension. Patients with neoatherosclerosis (versus without neoatherosclerosis) had a higher rate of target lesion revascularisation (92.6% vs. 77.6%, respectively, p=0.018) and stent thrombosis (14.8% vs. 0%, respectively, p<0.001).
Conclusions: Neoatherosclerosis occurred in one-third of stented lesions with >50% percent CSA stenosis of neointima. Late-phase development of neoatherosclerosis might be associated with clinical deterioration of stented lesions.
Introduction
The phenomenon of atheromatous changes within neointimal tissue, namely neoatherosclerosis, is uncommon in bare metal stents (BMS) and is considered to occur more than five years post BMS implantation1-4. Conversely, previous pathologic studies have reported a more frequent and accelerated occurrence of neoatherosclerosis in drug-eluting stent (DES)-treated lesions compared to BMS-treated lesions4,5. Several in vivo optical coherence tomography (OCT) studies have also demonstrated an earlier and more frequent presentation of neoatherosclerosis in DES-treated versus BMS-treated lesions6-8. Therefore, neoatherosclerosis may be an important mechanism of DES failure9.
Nevertheless, detailed in vivo information about neoatherosclerosis is limited, in that previous OCT studies were performed in small numbers of patients with variable symptoms or small numbers of lesions with variable amounts of neointimal tissue6-8. Therefore, the present OCT study was designed to investigate the time course, risk factors and clinical implication of neoatherosclerosis in a large number of mostly symptomatic patients with a significant burden of neointimal tissue.
Methods
STUDY DESIGN AND PATIENTS
From our institute’s OCT registry database, we identified 158 consecutive patients with >50% neointimal cross-sectional area (CSA) stenosis who were studied between January 2008 and July 2012. Among these patients, six patients were excluded, five due to poor OCT image quality, and one because of balloon angioplasty before acquiring OCT images. Consequently, a total of 152 patients with 128 DES- and 24 BMS-treated lesions were included in this study. The median time intervals from stent implantation to follow-up OCT were 22.4 (11.6-51.3) months in DESs and 130.4 (110.2-159.7) months in BMSs. The reasons for follow-up angiography were: 1) evidence of myocardial ischaemia or symptoms of coronary artery disease (n=126); or 2) planned follow-up angiography for other stented lesions (n=26). The selection of DES at the time of coronary intervention was at the physician’s discretion; patients were treated with 41 sirolimus-eluting stents (CYPHER®; Cordis, Johnson & Johnson, Warren, NJ, USA), 33 paclitaxel-eluting stents (TAXUS®; Boston Scientific, Natick, MA, USA), 29 zotarolimus-eluting stents (Endeavor® Sprint or Endeavor® Resolute®; Medtronic, Minneapolis, MN, USA), 16 everolimus-eluting stents (XIENCE®; Abbott Vascular, Abbott Park, IL, USA), and nine biolimus-eluting stents (Nobori®; Terumo Corporation, Tokyo, Japan). BMS and DES implantation was performed using conventional techniques. Unfractionated heparin was administered as an initial bolus of 100 IU/kg, with additional boluses administered during the procedure to achieve an activated clotting time of 250 to 300 seconds. Dual antiplatelet therapy (aspirin and clopidogrel) was recommended to all patients for at least 12 months after DES implantation, and for one month after BMS implantation. This study was approved by the Institutional Review Board of our institute, and written informed consent was obtained from each patient. At the time of OCT follow-up, the clinical presentations of the patients were acute coronary syndrome in 21 patients, stable angina in 105 patients, and no symptoms in 26 patients. Eight patients with stent thrombosis were included in the group of 21 patients with acute coronary syndrome. General inclusion and exclusion criteria of follow-up OCT procedures have been previously reported10.
Stent thrombosis was defined according to the recommendations of the Academic Research Consortium11. The timing of stent thrombosis was classified as late (31-365 days) or very late (more than 366 days) post index procedure. Target lesion revascularisation (TLR) was defined as a repeat percutaneous intervention or bypass surgery of the target lesions with the following findings: ischaemic symptoms or positive stress test and angiographic minimal lumen diameter stenosis ≥50% by quantitative coronary angiographic analysis or angiographic diameter stenosis ≥70% by quantitative coronary angiographic analysis without either ischaemic symptoms or a positive stress test.
OCT PROCEDURE AND ANALYSIS
OCT was performed with either the Model M2 imaging system or the C7-XR™ imaging system (LightLab Imaging, Inc., St. Jude Medical, St. Paul, MN, USA). In the former system (Model M2), the occlusion catheter was positioned proximal to the stent and a 0.014 inch wire-type imaging catheter was positioned distal to the stent. During image acquisition, the occlusion balloon (Helios; Avantec Vascular Corp., Sunnyvale, CA, USA) was inflated to 0.4 to 0.6 atm, and lactated Ringer’s solution was infused at 0.5 to 1.0 mL/sec. The imaging wire was pulled from distal to proximal with a motorised pullback system at 1.0 mm/sec10. The frequency-domain OCT system (Model C7-XR™) has been developed to generate frames at much higher rates and thus allow faster pullback speeds. OCT images were generated at 100 frames/sec while a catheter was pulled back at 20 mm/sec. A non-occlusive contrast medium was continuously flushed through a guiding catheter at a rate of 4 to 5 ml/sec for three to four seconds. With both systems, continuous images were acquired and stored digitally for subsequent analysis. OCT was performed immediately after successful and careful aspiration of thrombus in patients with intra-stent thrombus. When the attenuation caused by intraluminal material obscured the underlying neointima, the characteristics of neointima were identified proximal or distal to the intraluminal material.
All OCT images were analysed at a core laboratory (Cardiovascular Research Center, Seoul, South Korea) by analysts who were blinded to patient and procedural information. Cross-sectional OCT images were analysed at 1 mm intervals for quantitative measurement at the segment of >50% cross-sectional area stenosis of neointima: (neointimal CSA/stent CSA) ×100. Stent and luminal CSA were measured, and neointimal CSA was calculated as the stent CSA minus the luminal CSA. As shown in Figure 1, stented segments with >50% neointimal CSA stenosis were then assessed qualitatively to characterise neointimal tissue as: 1) homogeneous neointima, an uniform signal-rich band without focal variation or attenuation; 2) heterogeneous neointima, focally changing optical properties and various backscattering patterns; 3) layered neointima, layers with different optical properties, namely an adluminal high scattering layer and abluminal low scattering layer; and 4) neoatherosclerotic neointima that contains the following features - lipid-laden neointima, neointima with calcification or thin-cap fibroatheroma3,6,12,13. Lipid-laden neointima was defined as a signal-poor region with diffuse borders, and neointima with calcification was defined as a well-delineated, signal-poor region with sharp borders13. To distinguish layered neointima from lipid-laden neointima, we postulated that a signal-poor region that fully masked the stent struts behind the neointima was considered as lipid-laden neointima. The inter- and intra-observer agreement for assessment of neointimal tissue morphology in our laboratory has already been reported in a previous study14. A thin-cap fibroatheroma-like neointima was defined as neointima with a fibrous cap thickness at the thinnest part ≤65 µm and an angle of lipid-laden neointima ≥180°3,6,13. Neointimal rupture was a break in the fibrous cap that connected the lumen with the underlying lipid pool. Intraluminal material was defined as visible material inside the vessel lumen12. Microvessels were defined as well-delineated low backscattering structures <200 μm in diameter showing a trajectory within the vessel12. The criteria for the diagnosis of neoatherosclerosis were lesions with lipid-laden neointima, neointima with calcification, a thin-cap fibroatheroma-like neointima or neointimal rupture.
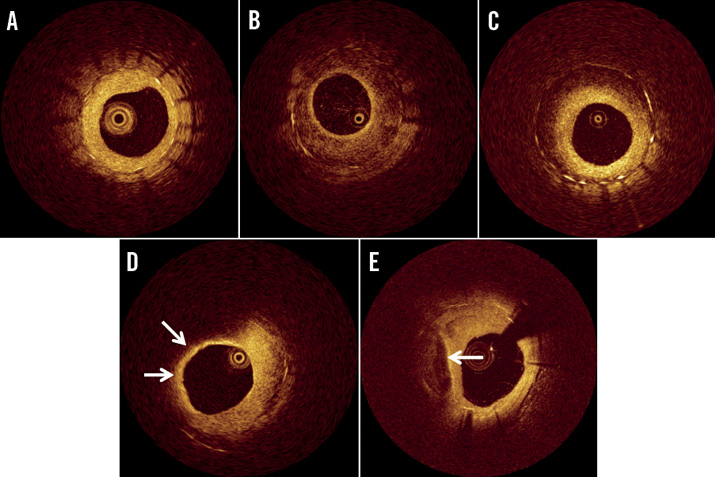
Figure 1. Representative optical coherence tomographic images of neointimal tissue. A) Homogeneous type, B) heterogeneous type, C) layered type, D) lipid-laden neointima (arrows), and E) neointima with calcification (arrow).
QUANTITATIVE ANGIOGRAPHIC ANALYSIS
Quantitative coronary angiographic analysis was performed using an offline computerised quantitative coronary angiographic system (CASS system; Pie Medical Imaging, Maastricht, The Netherlands) in an independent core laboratory (Cardiovascular Research Center, Seoul, South Korea). The minimal lumen diameter and reference diameters of treated coronary lesions were measured in the view with the narrowest lumen and the least amount of foreshortening.
STATISTICAL ANALYSIS
Statistical analysis was performed using PASW version 18.0.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as number (%) or as mean±standard deviation or median (interquartile range). Comparisons of categorical data were made using χ-square statistics or Fisher’s exact test. Continuous variables were compared using Student’s t-test, Mann-Whitney test, or Kruskal-Wallis test. Receiver-operating characteristic curves were analysed to assess the best cut-off values of the time predicting neoatherosclerosis with a maximal accuracy using MedCalc (MedCalc Software, Mariakerke, Belgium). Multiple logistic regression analysis was performed to determine the independent risk factors for neoatherosclerosis. The variables with a p-value <0.1 in univariate analysis (traditional cardiac risk factors such as diabetes mellitus, hypertension and hypercholesterolaemia) and procedural variables such as stent type, diameter, and length were entered into the multiple logistic regression model. A p-value <0.05 was considered statistically significant.
Results
Baseline characteristics are listed in Table 1. Neoatherosclerosis was observed in 54 lesions (35.5%) with 35 DESs and 19 BMSs (27.3% [35/128] in DES and 79.2% [19/24] in BMS). Among 21 patients who presented with acute coronary syndrome at follow-up OCT, neoatherosclerosis was observed in 17 patients (81.0%). Compared to patients without neoatherosclerosis, those with neoatherosclerosis more often required repeat TLR (77.6% vs. 92.6%, respectively, p=0.018) and more often presented with stent thrombosis (0% vs. 14.8%, respectively, p<0.001). Intraluminal material was more frequently observed in lesions with neoatherosclerosis than in lesions without neoatherosclerosis (Table 1). Among 126 lesions with repeat TLR, seven lesions were treated with plain old balloon angioplasty, 87 lesions with a drug-eluting balloon, and 32 lesions with DES.
The median time from stent implantation to follow-up OCT was 70.7 months in lesions with neoatherosclerosis, longer than lesions without neoatherosclerosis (13.4 months, p<0.001). The median time from stent implantation to follow-up OCT was 58.7 months in DES-treated lesions with neoatherosclerosis and 129.5 months in BMS-treated lesions with neoatherosclerosis (p<0.001). The best cut-off time of neoatherosclerosis in DES-treated lesions was 30 months with a sensitivity of 91.4% and a specificity of 72.0% (area under curve=0.839, 95% confidence interval [CI]: 0.764 to 0.898, p<0.001) (Figure 2). OCT findings in DES-treated lesions studied >30 months after implantation compared to those studied <30 months after implantation are shown in Table 2. The best cut-off time of neoatherosclerosis in BMS-treated lesions could not be calculated due to the small number of BMS-treated lesions. Figure 3 represents the time effect of neoatherosclerosis on TLR of 105 DES-treated lesions. The incidence of neoatherosclerosis increased from 10% (3/30) in patients who underwent TLR <12 months post intervention to 68.2% (15/22) in patients who underwent TLR >60 months post intervention (p<0.001).
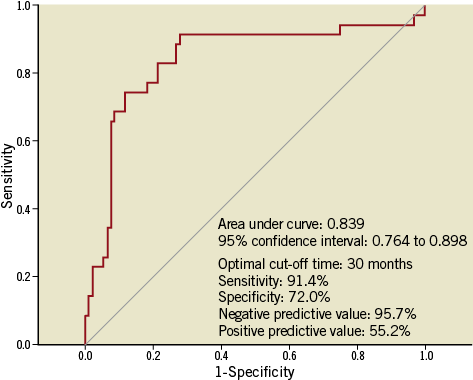
Figure 2. Receiver-operating characteristic curve analysis to determine the threshold of the follow-up time interval that predicted neoatherosclerosis in 128 drug-eluting stent-treated lesions.
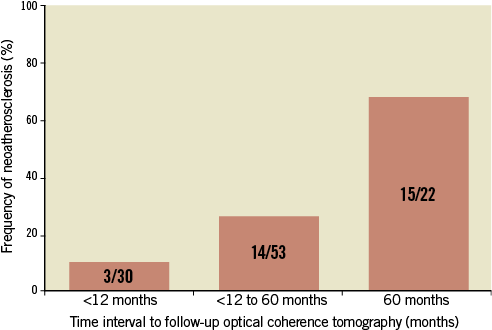
Figure 3. The time effect of neoatherosclerosis on TLR of 105 DES-treated lesions. Neoatherosclerosis was seen in 3/30 (10%) patients who underwent TLR within 12 months after DES implantation, in 14/53 (26.4%) patients who underwent TLR between 12 and 60 months after DES implantation, and in 15/22 (68.2%) patients who underwent TLR >60 months after DES implantation (p<0.0001).
The incidence of neoatherosclerosis was significantly higher in lesions treated with first-generation DESs (sirolimus- or paclitaxel-eluting stents) than in lesions treated with next-generation DESs (zotarolimus-, everolimus- or biolimus-eluting stents) (40.5% vs. 10.2%, respectively, p<0.001).
The independent risk factors for neoatherosclerosis were stent age (odds ratio [OR] 1.054, 95% CI: 1.028-1.081, p<0.001), use of first-generation DES (OR 14.666, 95% CI: 1.065-201.936, p=0.045) and hypertension (OR 4.223, 95% CI: 1.354-13.170, p=0.013) in multiple logistic regression analysis.
Discussion
This OCT study showed that neoatherosclerosis occurred in 35.5% of stented lesions with >50% neointimal CSA stenosis. The optimal cut-off time to predict neoatherosclerosis in DES-treated lesions was 30 months. Independent risk factors for neoatherosclerosis were stent age, use of first-generation DES and hypertension. Compared to patients without neoatherosclerosis, those with neoatherosclerosis more frequently underwent TLR or suffered from stent thrombosis.
In DES-treated lesions the incidence of neoatherosclerosis increased as the follow-up duration after stent implantation became longer. A previous pathologic study reported that the incidence of neoatherosclerosis was 29% in DES implanted for less than two years and 41% in DES implanted for more than two years4. In a recent OCT study the incidence of lipid-laden neointima increased from 37% in DES implanted for less than nine months, to 63% in DES that were implanted for between nine months and two years, and to 75% in DES that were implanted for more than two years7. These findings were similar to the findings in patients who underwent TLR in the present study in whom the incidence of neoatherosclerosis increased from 10% within 12 months after DES implantation, to 26.4% between 12 and 60 months after DES implantation, and to 68.2% more than 60 months after DES implantation (p<0.001). However, the time interval to detect neoatherosclerosis in the present study was longer than that in the ex vivo report (median 14 months)4 and the previous OCT study (mean 20.1 months)7. These differences may be due to the types of DES studied or the fact that we only studied lesions with >50% neointimal CSA stenosis. Another OCT study of 50 DES-treated lesions with >50% neointimal CSA stenosis reported that 20 months after DES implantation was the best threshold to predict the presence of thin-cap fibroatheroma-like neointima6. The optimal cut-off time to predict neoatherosclerosis in 128 DES-treated lesions was 30 months in the present study.
The possible mechanisms to enhance neoatherosclerosis inside DES were delayed coverage and dysfunction of endothelial cells which were barriers to inhibiting infiltration of lipids and migration of inflammatory cells5. In the process of neointimal healing, everolimus- or zotarolimus-eluting stents showed better endothelial strut coverage at 14 and 28 days after stent implantation in an animal model compared to first-generation DESs15. Recent clinical studies also reported more favourable clinical outcomes in patients treated with everolimus- or zotarolimus-eluting stents compared to first-generation DESs16,17. There have been no published data to report the incidence of neoatherosclerosis according to different types of DES. Compared to first-generation DESs, the incidence of neoatherosclerosis was lower in next-generation DESs in the present study.
Younger patient age, longer stent implant duration, use of DES over BMS, and underlying unstable lesions were identified as independent risk factors for neoatherosclerosis in a pathologic study4. A recent OCT study suggested that stent age (≥48 months), use of DES, current smoking, chronic kidney disease, and the use of angiotensin converting enzyme inhibitors or angiotensin II receptor blockers were independent risk factors for neoatherosclerosis8. As in these reports, stent age, use of DESs, and hypertension were independent risk factors for neoatherosclerosis in the present study.
In the present study, neoatherosclerosis (versus neointima without neoatherosclerosis) involved 81% of patients with acute coronary syndrome and 100% of patients with stent thrombosis, resulting in a higher incidence of intraluminal material (46.3% vs. 18.4%, respectively, p<0.001) and a higher rate of TLR (92.6% vs. 77.6%, respectively, p=0.018). These results were consistent with a previous OCT study in which patients presenting with unstable angina showed a higher incidence of OCT-defined thin-cap fibroatheroma-like neointima (75% vs. 37%, p=0.008) and thrombi (80% vs. 43%, p=0.010) than patients presenting with stable angina6. Thus, the development of neoatherosclerosis might be related to a clinically unstable presentation and seems to be the main mechanism of late stent complication. The findings that 81% of patients with acute coronary syndrome and 100% of patients with stent thrombosis had neoatherosclerosis in this study showed a higher incidence of neoatherosclerosis than a prior intravascular ultrasound study by Lee et al18. This difference is probably explained by different inclusion criteria (≤50% neointimal CSA stenosis in this study) and the better resolution and acuity offered by OCT (compared to intravascular ultrasound) to assess neoatherosclerosis.
Limitations
This study had several limitations. The study population was relatively small and there may have been some potential for selection bias. Direct comparisons between BMS and DES were not available due to the small number of BMS-treated lesions and the different time interval to follow-up between the two groups. Results from the present study suggest that next-generation DES might be superior to first-generation DES in respect of the development of neoatherosclerosis. However, further investigation should be mandatory because long-term OCT follow-up of next-generation DES was not yet available and there was a potential bias for the underrepresentation of the next-generation DES. The current OCT technology might have some limitations in the accurate assessment of neointimal tissue characteristics.
Conclusions
This OCT study suggested that neoatherosclerosis occurred in one-third of stented lesions with >50% CSA stenosis of neointima. Neoatherosclerosis might contribute to clinical deterioration of patients at a late phase after DES implantation. OCT could help to understand the mechanism of late stent complications and could drive the therapeutic option for in-stent restenosis.
Funding
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A085012 and A102064), a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A085136), and the Cardiovascular Research Center, Seoul, South Korea.
Conflict of interest statement
The authors have no conflicts of interest to declare.

