
Abstract
Aims: The aim of the study was to investigate the association between in-stent neoatherosclerosis (NA) and atherosclerosis progression (AP) in non-culprit segments in patients with ST-elevation myocardial infarction at five years.
Methods and results: Sixty-two out of 169 consecutive patients included in the EXAMINATION study underwent optical coherence tomography (OCT) at five years. NA plaques were observed in 13 (21.0%), signal-rich bands (SRB) in 22 (35.5%) and AP in 11 (17.7%). NA plaques were more frequently observed in patients treated with two stents (53.8% vs. 20.4%; p=0.02). SRB were more frequently observed with longer stent length (29.8±11.6 vs. 22.5±9.1 mm; p<0.01), larger stent size (3.4±0.4 vs. 3.1±0.4 mm; p<0.01) and with bare metal stents (BMS) (68.2% vs. 40.0%; p=0.03). Patients with AP had higher levels of LDL-cholesterol (108.3±27.1 vs. 86.3±27.6 mg/dl; p=0.02). QCA of 744 non-culprit segments showed no association between NA plaques or SRB and reduction of lumen diameters. By multivariate analysis, NA plaques were associated with stent length; SRB were associated with stent length and BMS. AP was associated with mean LDL-cholesterol levels.
Conclusions: NA and SRB had no association with AP or with LDL-cholesterol. NA and SRB were associated with stent-related factors such as stent length and BMS. AP was associated with LDL-cholesterol levels.
Abbreviations
AP: atherosclerosis progression
BMS: bare metal stents
NA: neoatherosclerosis
QCA: quantitative coronary angiography
SRB: signal-rich bands
SMC: smooth muscle cells
STEMI: ST-elevation myocardial infarction
Introduction
Coronary atherosclerosis is initiated by dysfunction of the endothelium. Endothelial dysfunction has been associated with all well-known cardiovascular risk factors (such as smoking, high blood pressure, hypercholesterolaemia and diabetes mellitus)1. Endothelial dysfunction reduces the synthesis of nitric oxide and favours a pro-inflammatory and a pro-coagulant state of the vessel wall. The inflammation process of the vessel wall causes the migration and accumulation of smooth muscle cells (SMC) within the hyaluronan and proteoglycan matrix, and causes the focal accumulation of extracellular lipid. This state is known as pathologic intimal thickening and is the first phase of coronary atherosclerosis. Macrophages may or may not participate in this early stage of atherosclerosis1.
The pathological mechanisms of neoatherosclerosis (NA) are still under investigation. Stent implantation causes denudation of the endothelium and injury of the media/adventitia. During the first days after stent implantation, the denuded endothelium regrows from the remaining endothelial cells and uninjured segments proximal and distal to the stent. Simultaneously, the SMC from the media migrate and accumulate above and beside the stent/struts forming the neointimal tissue. Therefore, the healing process after coronary stent implantation is similar to the pathologic intimal thickening observed in native coronary atherosclerosis. This process is delayed and reduced with drug-eluting stents (DES) compared to bare metal stents (BMS). According to pathology studies, in-stent NA is defined when lipid-laden foamy macrophages infiltrate the neointimal tissue2.
In both processes, the endothelium is dysfunctional and causes increased permeability to extracellular lipids and favours migration of inflammatory cells, such as macrophages, into the intima/neointima tissue. In native coronary arteries this process may take decades. However, it is accelerated in cases of NA (around 900 days after BMS implantation and 70 days after first-generation DES implantation)1,2.
The objective of the present study was to investigate the association between atherosclerosis progression (AP) of native coronary arteries and the emergence of in-stent NA in patients with ST-elevation myocardial infarction (STEMI) treated with stent implantation at five years from the stent implantation.
Methods
PATIENT POPULATION AND MEDICAL TREATMENT
The EXAMINATION (a clinical Evaluation of the XIENCE-V stent in Acute Myocardial INfArcTION) study is a randomised, controlled, all-comers, multicentre trial (NCT00828087). From 2008 to 2010, 1,504 patients with STEMI were randomised to one of the two treatment arms: everolimus-eluting stent (EES) (XIENCE V®; Abbott Vascular, Santa Clara, CA, USA) vs. BMS stent (MULTI-LINK VISION®; also Abbott Vascular). The design and results of the EXAMINATION study have been reported previously3.
For the purpose of the present substudy, all consecutive patients included in the EXAMINATION study from September 2009 to January 2010 in two participating institutions were screened to participate in the RE-EXAMINATION study five years after stent implantation4. A flow chart of the study is shown in Figure 1.

Figure 1. Flow chart of the study. OCT: optical coherence tomography; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; TLR: target lesion revascularisation
This study was approved by the local ethics committee of all participating institutions and was carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
QUANTITATIVE CORONARY ANGIOGRAPHY ANALYSIS
Coronary segments were defined according to the modified American Heart Association/American College of Cardiology (AHA/ACC) coronary segment classification. Similar angiographic views of non-culprit coronary segments were matched between baseline (BL) and five-year follow-up (FU)4. Coronary atherosclerosis progression (AP) between baseline and five-year FU was defined when one of the following criteria was met: a) increase of ≥10% diameter stenosis (DS) of at least one pre-existing lesion with ≥50% DS, b) increase of ≥30% DS of a pre-existing lesion with <50% DS; c) progression of a lesion to total occlusion from baseline to angiographic follow-up5. Figure 2 shows an example of the angiographic analysis used for AP assessment in non-culprit segments.
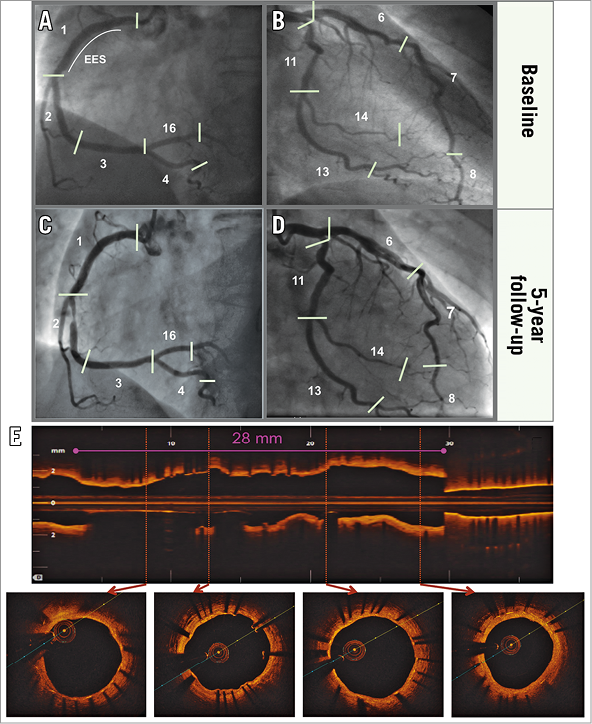
Figure 2. Angiographic assessment of atherosclerosis progression. A 67-year-old female patient with inferior ST-elevation myocardial infarction treated with an everolimus-eluting stent in the proximal right coronary artery (RCA). The patient remained asymptomatic and underwent five-year angiography and OCT imaging showing atherosclerosis progression in the mid-RCA and no signs of neoatherosclerosis. A) & B) Angiographic analysis of culprit and non-culprit segments at the end of the index procedure. C) & D) Analysis of matched segments at 5 years. E) 5-year OCT imaging..
OPTICAL COHERENCE TOMOGRAPHY (OCT) ANALYSIS
OCT analysis was performed by a dedicated imaging core laboratory (BARCICORE-lab, Barcelona, Spain) using specific software (LightLab Imaging, Westford, MA, USA). Two blinded analysts were requested to assess in-stent NA plaques or signal-rich bands in the entire OCT pullback (0.2 mm intervals) of the stented segment at five-year FU4. Neoatherosclerotic plaques were defined as the observation of a fibroatheroma or fibrocalcified cores within the neointima with a longitudinal extension ≥1 mm. Fibroatheromatous plaques were characterised as signal-poor regions with high signal attenuation and diffuse borders. Fibrocalcified plaques were defined as signal-poor regions with low signal attenuation and clear plaque borders6. Signal-rich bands (SRB) were defined as hyper-bright lines with important signal attenuation6. Figure 3 shows cases with normal neointimal tissue, NA plaques and SRB.
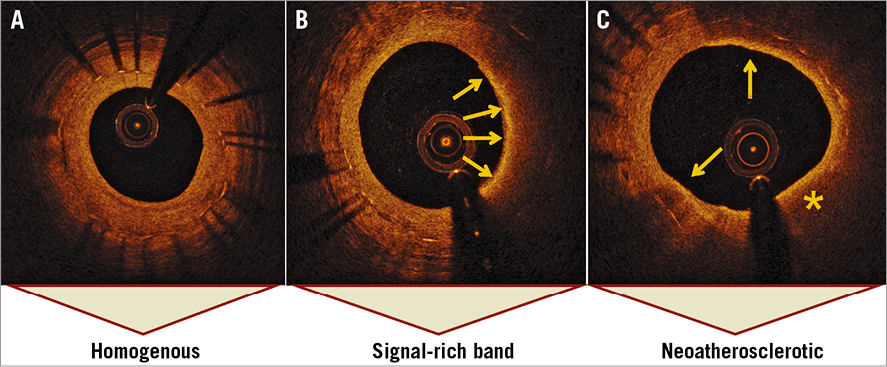
Figure 3. Optical coherence tomography characterisation of in-stent neoatherosclerosis. A) Homogeneous neointima composed by smooth muscle cells. B) Signal-rich bands are defined as hyper-bright structures with high signal attenuation (arrows). The most probable component is foamy macrophages (57-68%). C) Lipid-rich neoatherosclerotic plaque (asterisk) defined as a signal-poor structure with high signal attenuation surrounded by signal-rich bands (arrows).
STATISTICAL ANALYSIS
All patients included in the present study were divided into three categories: 1) patients with and without in-stent NA plaques; 2) patients with and without in-stent SRB according to the five-year OCT images of the culprit segment; and 3) patients with and without AP according to the angiographic analysis of the non-culprit segments between BL and five-year FU. Categorical variables were presented as counts and percentages, and continuous variables as means±1 standard deviation. Comparisons of continuous variables between groups were estimated using the Student’s t-test for independent variables. Comparisons of continuous variables between matched BL and FU parameters were performed using the Student’s t-test for paired variables. Tests and comparisons of categorical variables were estimated with the chi-square test. Generalised estimating equation models were performed to associate predictive factors of NA, SRB and AP. Each of the three categorical variables was entered into the three models as a dependent variable for binary logistic response. All predictors related with the dependent variable in the univariate analysis with a p-value <0.15 were included in each model as factors (categorical variables) or covariates (continuous data). A two-sided p-value ≤0.05 was considered statistically significant. Statistical analysis was performed with SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
CLINICAL CHARACTERISTICS AT FIVE YEARS
A total of 62 out of 169 patients screened to participate in the present study were included. Figure 1 shows the study flow chart. There were 13 patients (21.0%) with in-stent NA, 22 patients (35.5%) with SRB, and 11 patients (17.7%) with AP in non-culprit segments between BL and five-year FU. NA plaques were observed in one patient (9.1%) with AP and in 12 patients (23.5%) without AP (p=0.286). SRB were observed in two patients (18.2%) with AP and in 20 patients (39.2%) without AP (p=0.186). SRB were observed in all patients with NA plaques (100%).
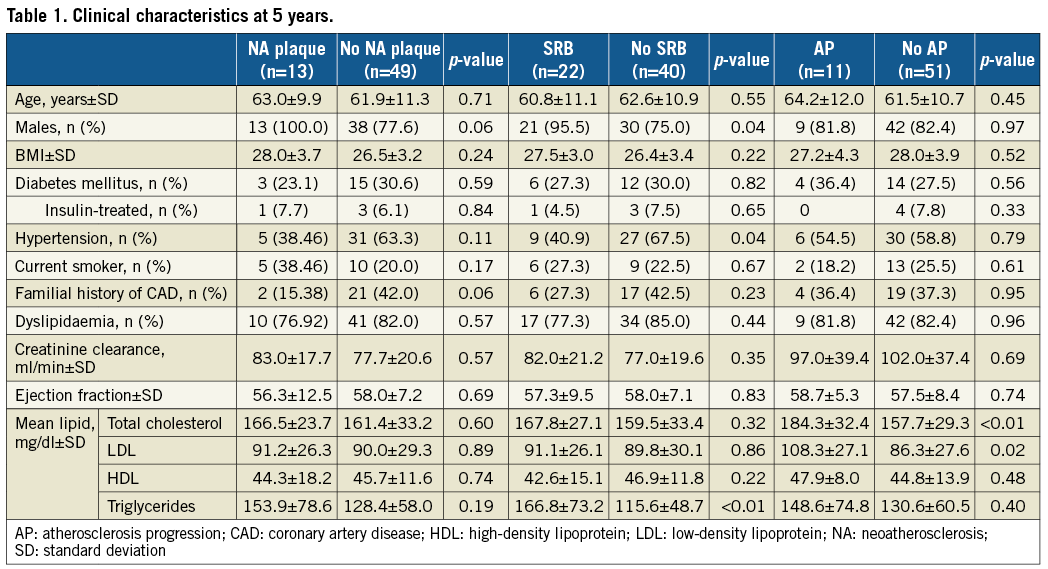
Table 1 shows the clinical characteristics and lipid profile at five years. NA plaques (100 vs. 77.6%; p=0.06) and SRB (95.5 vs. 75.0%; p=0.04) were more frequent in males. Mean low-density lipoprotein (LDL) cholesterol was similar in patients with and without NA plaques (91.2±26.3 vs. 90.0±29.3 mg/dl; p=0.89) and SRB (91.1±26.1 vs. 89.8±30.1 mg/dl; p=0.86), but was higher in AP subjects (108.3±27.1 vs. 86.3±27.6 mg/dl; p=0.02).
PROCEDURAL CHARACTERISTICS AND QCA ANALYSIS IN CULPRIT (STENT) SEGMENTS
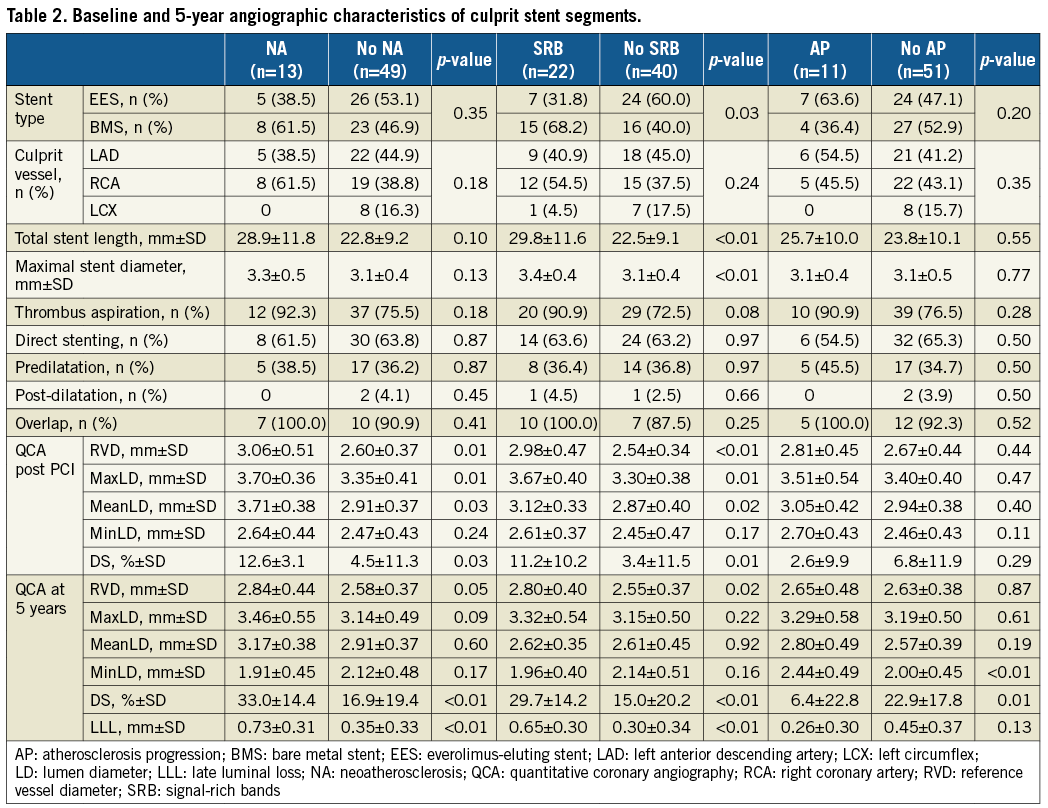
Table 2 shows the baseline and five-year angiographic characteristics of the culprit stent segments. Patients with NA plaques and SRB were treated with a longer stent length (28.9±11.8 and 29.8±11.6 mm) vs. patients without (22.8±9.2 and 22.5±9.1 mm; p=0.10 and <0.01, respectively). Patients with SRB were also treated with a larger stent size (3.4±0.4 vs. 3.1±0.4 mm; p=0.003) and more frequent use of BMS (68.2% vs. 40.0%; p=0.03) than in patients without. Post-PCI QCA analysis showed larger values of RVD in patients with NA plaques (3.06±0.51 vs. 2.60±0.37 mm; p=0.01) and patients with SRB (2.98±0.47 vs. 2.54±0.34 mm; p<0.01) than in patients without. Residual DS was also larger in patients with NA plaques (12.6±3.1 vs. 4.5±11.3%; p=0.03) and in patients with SRB (11.2±10.2 vs. 3.4±11.5%; p=0.01) than in patients without. At five-year FU, patients with AP presented with larger minimal lumen diameter (MinLD) (2.44±0.49 vs. 2.00±0.45 mm; p<0.01) and lower DS (6.4±22.8 vs. 22.9±17.8%; p=0.01) than patients without AP.
QCA IN NON-CULPRIT (NON-STENT) SEGMENTS
A total of 778 non-culprit matched segments were screened for AP analysis between the index procedure and five-year FU. Table 3 shows the quantitative coronary angiography analysis of the 744 matched segments. All subgroups presented with significant reductions of lumen diameters between the index procedure and five-year control. These reductions in lumen diameter were similar between patients with and without NA and SRB. However, patients with AP presented with a larger reduction of MinLD (–0.17±0.65 vs. –0.04±0.37 mm; p=0.06) and greater increase of DS (7.8±26.9 vs. –3.8±14.1%; p<0.01) as compared with patients without. Figure 4 shows the angiographic changes between baseline and five-year angiography in non-culprit segments.
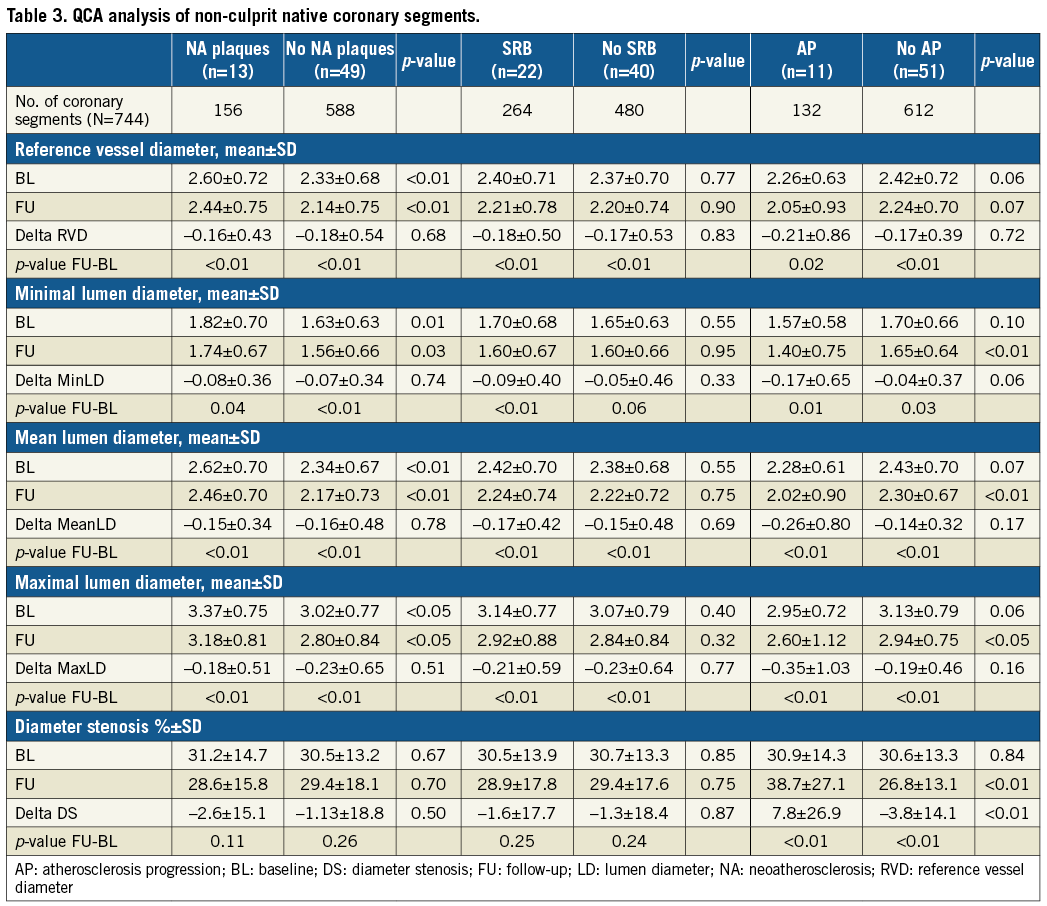
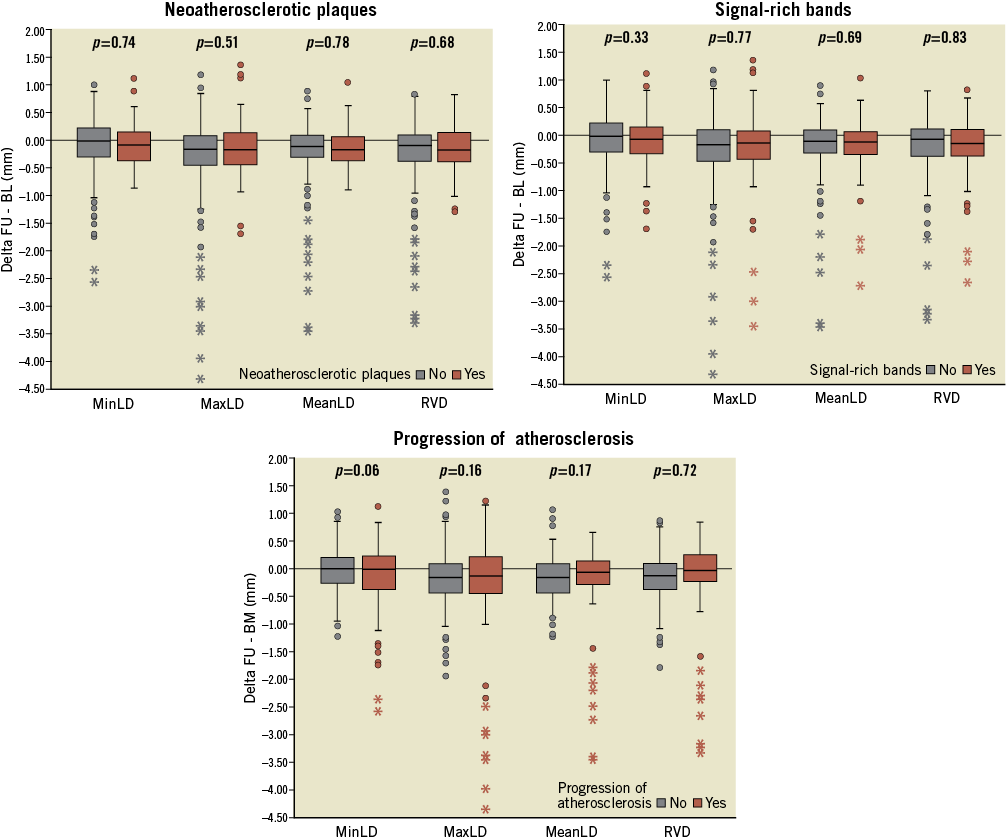
Figure 4. Angiographic lumen changes in non-culprit segments. MaxLD: maximal lumen diameter; MeanLD: mean lumen diameter; MinLD: minimal lumen diameter; RVD: reference vessel diameter
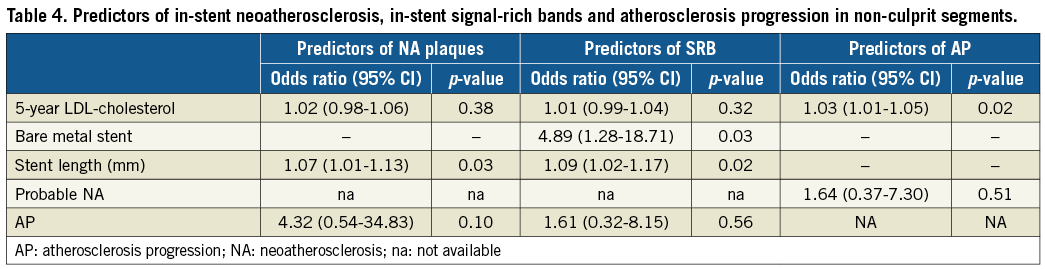
MULTIVARIATE PREDICTORS OF NA, SRB AND AP
Table 4 shows the multivariate predictors of NA plaques, SRB and AP. Stent length was the only independent predictor of NA plaques with an odds ratio (OR) of 1.07 (95% confidence interval [CI]: 1.01-1.13). Use of BMS (OR 4.89, 95% CI: 1.28-18.71) and stent length (OR 1.09, 95% CI: 1.02-1.17) were independent predictors of SRB. Mean LDL-cholesterol level was the single independent predictor of AP.
Discussion
The main findings of the present study are: 1) NA plaques, SRB and AP were observed in 21%, 36% and 18%, respectively, of STEMI patients at five years; 2) NA plaques and SRB were more frequently observed in STEMI patients receiving BMS and in those with longer total stent length; 3) in contrast, AP was associated with high levels of LDL-cholesterol; and 4) finally, NA and SRB were not associated with AP or with mean LDL-cholesterol levels.
NA has been described as one of the major causes of very late stent thrombosis (ST) and restenosis7-9. According to OCT studies, NA with lipid-core or calcium-core plaques was observed in 14-63% of patients with DES and in 36-100% of patients with BMS very late ST7,8,10. However, little is known about the prevalence of NA and SRB in event-free patients. The study of Taniwaki et al included 88 patients treated with first-generation DES and imaged with OCT at five years. In this study, NA plaques and SRB were observed in 4.9% and 14.6% of patients treated with sirolimus-eluting stents (SES), and in 25.5% and 46.8% of patients treated with paclitaxel-eluting stents (PES), respectively6. The present study observed NA plaques and SRB in 16.1% and 22.5% of patients treated with EES, and in 25.8% and 48.4% of patients treated with BMS, respectively. Taking into account the mean neointima thickness of those stents as assessed by OCT (BMS >PES >EES >SES)11, it is possible that stents with greater neointima response may be prone to a higher risk of NA plaques and SRB.
Moreover, NA has been associated with the lack of statin treatment and with high levels of LDL-cholesterol in small observational studies12-14. However, similar studies have not found these associations between NA and statin treatment or with LDL-cholesterol levels6,15. The study of Taniwaki et al did not find this association between NA and LDL-cholesterol levels, but found a larger reduction of the angiographic MinLD of non-culprit native coronary segments in patients with in-stent NA (–0.25 mm) than in patients without in-stent NA (–0.13 mm) (p=0.002)6. The authors concluded that the mechanisms involved in the progression of the native coronary artery disease in non-culprit segments might have similarities to the pathophysiologic mechanisms of in-stent NA. In the present study, the reduction of the MinLD in non-culprit native coronary segments was similar between patients with in-stent NA (–0.08 mm) and patients without in-stent NA (–0.07 mm) (p=0.74). It is noteworthy that both studies found statistically significant reductions of the lumen diameters between the index procedure and five years in all patients irrespective of the observation of in-stent NA. This phenomenon can be caused by the natural history of coronary artery disease despite treatment with optimal current medications. According to serial IVUS studies, only intensive lipid treatments achieve stabilisation or mild reduction of coronary plaque burden16. In order to overcome this limitation, the present study defined AP as a binary variable according to the progression of the diameter stenosis in matched non-culprit segments5. Moreover, the present study only included patients with STEMI. It is well known that patients with STEMI usually have vasoconstriction of the culprit and non-culprit vessels. For this reason, a restrictive definition of AP (as assessed by the increase of the angiographic DS between baseline and five years) seems more accurate than MinLD changes in this group of patients. In the present study, most of the patients (45.5%) required PCI of new angiographic lesions or presented with chronic total occlusions of small vessels that were left for medical treatment (18.2%).
Although OCT has excellent image resolution, the assessment of NA is still unclear. According to pathology studies, NA is defined as the accumulation of lipid-laden foamy macrophages with or without necrotic-core formation and/or calcification within the neointima17. Macrophages are defined as signal-rich, distinct, or confluent punctate regions that exceed the intensity of background speckle noise and can cause shadowing of underlying tissue structure as assessed by OCT18. However, the observation of SRB by OCT has been associated with foamy macrophages in only 68% of cases18. Other causes of SRB were cholesterol clefts (7%), calcification (11%), elastic fibres (12%) and others (3%)18. For this reason, the present study divided patients with neoatherosclerotic plaques and patients with SRB. Patients with clear OCT fibroatheroma or fibrocalcified cores with a longitudinal extension ≥1 mm might have a high probability of neoatherosclerosis. In contrast, patients with SRB, as assessed by OCT, have only a moderate probability of neoatherosclerosis18. It is also noteworthy that all patients classified as having neoatherosclerotic plaques also have SRB.
Limitations
First, there are only a few patients with NA plaques, SRB and AP. Second, only 68% of signal-rich bands as assessed by OCT correlate with foamy macrophages as assessed by histopathology. Third, AP was defined by the changes in coronary lumen diameter between the index procedure and five-year FU as assessed by QCA. According to the Glagov phenomenon, lumen reductions are observed at the final stage of coronary disease. Therefore, it is possible that cases with important plaque growing (and therefore with AP) were unseen in the present study. Moreover, it is also probable that the use of two different imaging techniques to assess the three objectives of the study may have led to a remarkable disconnection of the reported findings. Finally, the clinical relevance of these OCT observations warrants long-term follow-up. The relationship between NA and cardiac events is still under investigation. Moreover, it is uncertain if new NA plaques can emerge beyond five years from stent implantation. Therefore, all conclusions of the present study are merely hypothesis-generating.
Conclusions
The observation of either NA plaques or SRB in stents implanted in culprit STEMI lesions is not associated with the lipid profile or AP in non-culprit segments at five years from the primary procedure. The causes of NA and SRB are related to stent factors (such as BMS implantation and stent length). In contrast, AP is related to lipid profile during the five years after the stent implantation.
| Impact on daily practice Neoatherosclerosis (NA) is one of the main causes of stent failure. There are two potential, non-exclusive, causes of in-stent NA –stent-related factors and patient/lesion-related factors. The present study failed to find consistent associations between NA and patient-related factors, but found a potential relationship between stent-related factors and NA. Further investigations should focus on stent design features to prevent the emergence of NA plaques. |
Funding
The study was funded by a grant from the Spanish Society of Cardiology.
Conflict of interest statement
The authors have no conflicts of interest to declare.

