
The incidence of very late stent thrombosis (VLST) after second-generation drug-eluting stent (DES) implantation has decreased compared to that previously seen after first-generation DES implantation. However, VLST remains a major concern and its mechanism has not yet been fully elucidated.
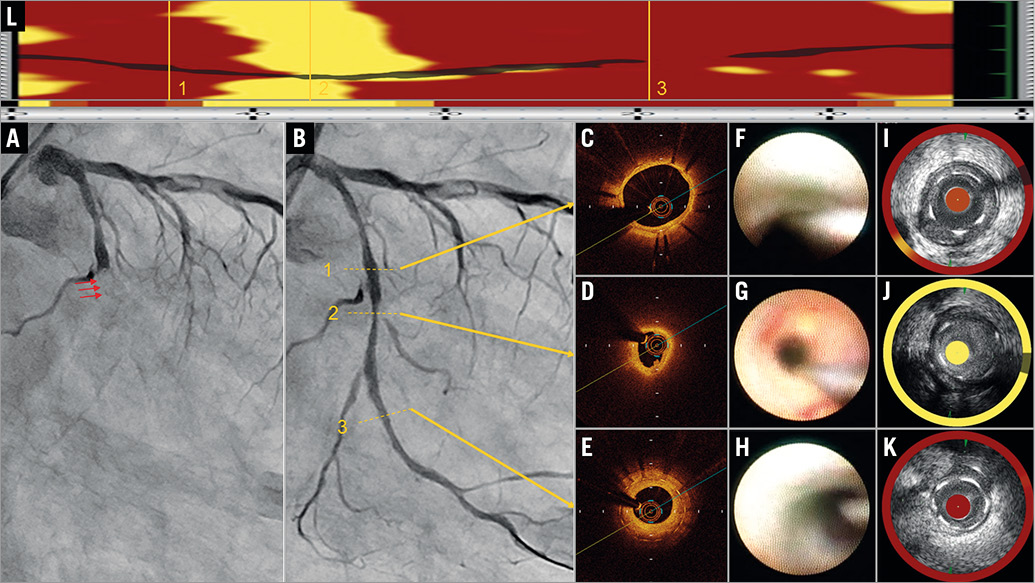
A 69-year-old man with angina pectoris was implanted with a durable polymer everolimus-eluting stent (EES) (XIENCE, 3.25×33 mm; Abbott Vascular, Santa Clara, CA, USA) in the left circumflex artery. At one-year follow-up, coronary angiography (CAG) demonstrated the patency of the EES. Dual antiplatelet therapy was switched to single antiplatelet therapy using clopidogrel. However, the patient had sudden onset of chest pain 26 months after the implantation. Emergent CAG revealed total occlusion of the EES site, which was attributed to very late stent thrombosis (VLST) (Panel A). Percutaneous coronary intervention was subsequently carried out and, following thrombus aspiration, complete recovery of blood flow was achieved (Panel B). On pathologic evaluation, most of the aspirated contents were composed of fresh red thrombi, and neutrophils, lymphocytes and a few macrophages without any atheromatous material. Optical coherence tomography (OCT) showed a lipid-rich intima without plaque rupture, and a thrombus with intact fibrous cap (Panel C-Panel E, Moving image 1). On angioscopy, a yellow plaque with red thrombus was observed (Panel F-Panel H). Lipid core burden index at the 4 mm maximal segment (LCBI4mm) evaluated by near-infrared spectroscopy (NIRS) was 997 (Panel I-Panel L). Drug-eluting balloon dilatation provided an excellent angiographic result.
Judging from intravascular images, no plaque rupture was detected although neoatherosclerosis was apparent when seen from a signal-poor region with diffuse borders between the lumen and the stent struts on OCT, yellow plaque on angioscopy and a very high LCBI4mm value evaluated by NIRS1. Previous OCT reports have shown that malapposition, neoatherosclerosis, uncovered struts and stent underexpansion are related to VLST2. A pathological review article has shown that neoatherosclerosis may eventually lead to in-stent plaque rupture3. Therefore, in cases without uncovered struts, underexpansion or malapposition, the cause of VLST is likely to be in-stent plaque rupture resulting from neoatherosclerosis. However, in this case, no plaque rupture was observed in the stented segment by OCT even though intravascular images apparently demonstrated neoatherosclerosis. We could diagnose this case as definite OCT erosion, which was identified by the presence of attached thrombus overlying an intact and visualised plaque4. Therefore, in-stent erosion is thought to be the cause of VLST. Furthermore, in this case, in-stent restenosis leading to critical deterioration of flow may have been one of the mechanisms contributing to VLST.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Optical coherence tomography immediately after thrombus aspiration at the time of percutaneous coronary intervention for very late stent thrombosis of a durable polymer everolimus-eluting stent. In the middle of the in-stent lesion, optical coherence tomography revealed a lipid-rich intima without plaque rupture and thrombus with an intact fibrous cap.
Supplementary data
To read the full content of this article, please download the PDF.
Optical coherence tomography immediately after thrombus aspiration at the time of percutaneous coronary intervention for very late stent thrombosis of a durable polymer everolimuseluting stent. In the middle of the in-stent lesion, optical coherence tomography revealed a lipid-rich intima without plaque rupture and thrombus with an intact fibrous cap.

