Abstract
Aims: To compare tissue coverage in coronary lesions stented with durable fluoropolymer-coated everolimus-eluting stents (EES) vs. biodegradable polymer-coated biolimus A9-eluting stents (BES).
Methods and results: Sixty-four patients (64 lesions) with de novo coronary artery lesions were randomised to percutaneous treatment with XIENCE® EES (Abbott Vascular, Santa Clara, CA, USA) vs. BioMatrix™ BES (Biosensors, Morges, Switzerland). The primary endpoint was the percentage of uncovered struts, as assessed with OCT, at nine months. The average percentage of uncovered struts was significantly lower with EES (4.3±4.8% vs. 8.7±7.8% with BES, p=0.019). There was no difference in the average percentage of malapposed struts at baseline (6.8±6.9% vs. 6.9±7.0%, respectively, p=0.974) and at follow-up (0.1±0.3% vs. 0.6±1.3%, p=0.143). Neointimal thickness at nine months was 109±43 µm in EES vs. 64±18 µm in BES (p<0.001), and angiographic LLL was 0.15 mm in EES vs. 0.10 mm in BES (p=0.581). We did not observe differences in the incidence of MACE and ST.
Conclusions: A significantly higher percentage of uncovered struts was detected in the BioMatrix BES compared with the XIENCE EES at nine-month follow-up. Our findings do not support a preferential use of stents with biodegradable polymer-based biolimus elution to reduce the risk for ST.
Introduction
Initial enthusiasm with regard to drug-eluting stents (DES) was tempered after an increased risk of late and very late stent thrombosis, associated with a delayed and incomplete coverage of stent struts, was observed in human pathology studies1,2. Too potent antiproliferative drugs and dosages as well as induction of hypersensitivity reactions to the polymer coating predominantly on first-generation DES have been held responsible for this delayed healing reaction. Subsequent DES development focused on altered stent platforms, the use of alternative antiproliferative limus analogues and the introduction of biocompatible and biodegradable polymers.
The biodegradable polymer biolimus A9-eluting stent was evaluated in the LEADERS trial, demonstrating favourable efficacy and long-term clinical safety3,4. In the LEADERS OCT substudy, improved arterial healing characteristics were reported, as compared to first-generation sirolimus-eluting stents5. Whether this advantage persists when compared to the cobalt-chromium everolimus-eluting stent (EES), for which a superior safety profile has been demonstrated in different studies and which is currently considered the standard comparator drug-eluting stent, remains to be proven. There is little information on the difference between arterial healing characteristics among different types of second-generation DES. Stent strut coverage as studied with optical coherence tomography (OCT) is considered a valuable surrogate marker for vessel healing after drug-eluting stent implantation2,6. Therefore, we conducted a randomised study (STACCATO) between the everolimus-eluting XIENCE® stent (Abbott Vascular, Santa Clara, CA, USA) and the biolimus A9-eluting BioMatrix™ stent (Biosensors, Morges, Switzerland), using stent strut coverage as assessed with optical coherence tomography (OCT) as a surrogate marker to assess midterm arterial healing.
Methods
The STACCATO study (Assessment of Stent sTrut Apposition and Coverage in Coronary ArTeries with Optical coherence tomography in patients with STEMI, NSTEMI and stable/unstable angina undergoing everolimus vs. biolimus A9-eluting stent implantation) is a single-centre, prospective, randomised, open-label trial.
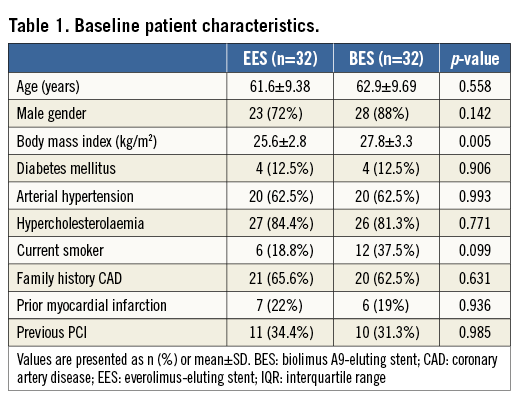
PATIENT POPULATION AND TREATMENT
Patients with either an acute coronary syndrome (ACS) or stable disease and with a de novo lesion <25 mm in length with a target reference vessel diameter between 2 and 4 mm could be included in the study. Exclusion criteria comprised a left ventricular ejection fraction of <30%, haemodynamic instability, impaired renal function (serum creatinine >2.0 mg/dL), significant left main coronary artery disease, extreme tortuosity or heavy calcification of the involved artery, prior invasive treatment of the culprit vessel, a target lesion located in a bifurcation, and known allergies to antiplatelet, anticoagulation therapy, contrast media, everolimus or biolimus A9.
The randomisation process was organised via an independent entity (Leuven Coordinating Centre, Leuven, Belgium), using an interactive voice response system (IVRS) and a centralised computer-generated random sequence. For practical reasons, the operator and the patient were not blinded to the treatment allocation.
TECHNICAL SPECIFICATIONS OF STUDY STENTS
XIENCE V®/XIENCE PRIME® EES (Abbott Vascular) are coated with a durable biocompatible fluoropolymer, designed to release 80% of the everolimus in the first 30 days after deployment7. BioMatrix BES carry a biodegradable polylactic acid polymer coating, which is gradually degraded into carbon dioxide and water. Biolimus A9 is released during the degradation process, which is completed after nine months3. The latter coating (and hence drug elution) is limited to the abluminal side of the stent, with the aim of favouring endothelialisation of the stent.
ENDPOINTS
The primary endpoint of the study was the percentage of uncovered stent struts at nine-month follow-up, as assessed with OCT. No formal sample size calculation was performed, given the lack of estimation of the magnitude of the possible difference in strut coverage between the two devices at the time the trial was designed. Based on previous OCT studies investigating healing in DES, a number of 64 patients was considered a reasonable estimate to detect meaningful differences in view of the stratification according to clinical presentation8.
Secondary endpoints were stent apposition (immediately after implantation and at follow-up) and neointimal thickness at follow-up, both assessed with OCT, and angiographic in-stent and in-segment minimum lumen diameter (MLD), late lumen loss (LLL), percentage diameter stenosis (% DS), and binary in-stent and in-segment restenosis.
A pre-specified secondary analysis equally included identical OCT endpoints according to the clinical presentation, independently of the type of stent used.
The study complies with the Declaration of Helsinki, was approved by the institutional ethics committee and registered at ClinicalTrials.gov (study identifier: NCT1065519). All patients provided written informed consent for participation in this trial.
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA) MEASUREMENTS
Digital coronary angiograms were analysed offline by the local core laboratory, using a validated automated edge detection system (ACOM.PC 5.0; Siemens AG, Munich, Germany). The MLD and % DS were evaluated at the end of the procedure and at follow-up both in the stent and in the stented segment (defined as the whole stented tract plus the 5 mm edges proximal and distal to the stent). LLL was calculated as the difference in MLD between measurements immediately after the procedure and at follow-up. Binary angiographic restenosis was defined as DS >50% by QCA on the follow-up angiogram.
OCT IMAGE ACQUISITION
OCT was performed using the frequency-domain OCT system (C7-XR™ Intravascular Imaging System and Dragonfly™ OCT catheter; St. Jude Medical, St. Paul, MN, USA) with a pullback speed of 20 mm/s and rotation speed of 100 frames/s, using a non-occlusive technique. OCT imaging was performed immediately after stent implantation and during the nine-month control angiography. Intracoronary administration of nitrates (100 to 300 µg) was performed before starting the intracoronary imaging procedure. After advancing the OCT catheter beyond the stented segment, an automatic pullback was started approximately 5 mm distally from the most distal stent struts as soon as the lumen was cleared of blood using an automated flush with contrast medium, to ensure appropriate image acquisition.
OCT ANALYSIS METHODOLOGY
A manual check of image quality and completeness of data was performed prior to the core lab analysis. Pullbacks or frames with poor image quality (mainly caused by residual blood, artefact or reverberation, or with portions of the stent out of the screen) were excluded from further analysis. Calculation of lumen and stent areas and quantitative strut level analyses were performed every three frames (i.e., approximately every 0.6 mm). The centre of the luminal surface of the strut was determined for each strut, and its distance to the lumen contour was calculated automatically to determine strut-level intimal thickness. Quantitative and qualitative OCT analyses were performed offline by the local core laboratory. The OCT Detecting Instrument for StEnt Reendothelialisation aNd Apposition (ODIERNA, Leuven, Belgium) was used for automatic detection of struts, strut coverage at follow-up and quantification of lumen area and stent area. Details of the algorithm and its validation against manual assessment and histopathology have been published earlier9,10. In the follow-up pullbacks, a strut was considered uncovered if any part of the strut was visibly exposed to the lumen. The thresholds for malapposition distance were calculated as the strut thickness+half of the blooming artefact (18 µm)11. For the XIENCE stent, with a strut thickness of 89 µm including a permanent polymer, it was set at 110 µm. For the BioMatrix stent, the strut thicknesses are 136 µm at baseline and 125 µm after degradation of the polymer. Cut-off values for malapposition were therefore set at 160 µm at baseline and 150 µm in the follow-up examinations. In the baseline analysis, struts overlying a side branch (SOB) were categorised separately and not reported as malapposed struts. OCT analysis was performed blinded for assessment of coverage and, given different cut-off values in both groups, unblinded for apposition assessment. Automatic measurements were supervised by expert analysts and manually edited if required. The OCT core lab assessment procedure is described in Figure 1.
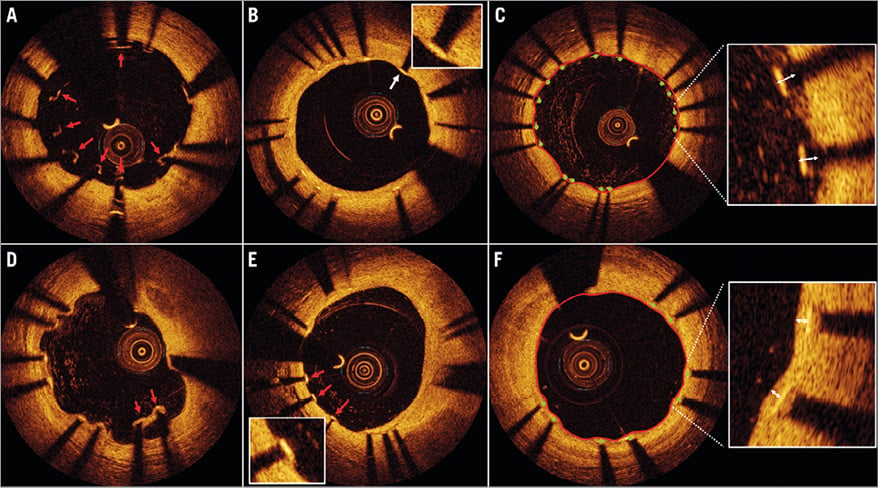
Figure 1. OCT analysis of stent apposition and coverage. A) OCT immediately after implantation. Red arrows indicate malapposed struts. B) OCT at nine months after DES implantation. All struts are well apposed and covered with a thin layer of neointima. C) Illustration of the ODIERNA software programme for the automated analysis of stent strut apposition. After delineation of the lumen contour (red line), automatic stent strut detection is achieved (green dots at the top of the stent struts) and malapposition distance can be calculated. D) Red arrows indicate malapposed struts in a DES at nine-month follow-up. E) Heterogeneous healing of stent struts at nine-month follow-up, with red arrows indicating uncovered struts, while the other struts in this frame are covered with a healthy layer of neointima. F) Automated analysis of neointimal coverage of stent struts. OCT: optical coherence tomography
STATISTICAL ANALYSIS
All analyses were performed according to the intention-to-treat (ITT) principle. Summary statistics are given per randomised treatment group. It is important to consider that, for each patient enrolled in the study, there was only one pre-specified lesion considered the target lesion. Therefore, there is no difference between patient level and lesion level analysis in this study. For continuous measurements, the number of observations with non-missing data, means and standard deviations (SD) or medians and interquartile ranges (IQR) are presented, where appropriate. For categorical variables, the observed frequencies and percentages are reported. For all continuous data (including OCT and QCA endpoints), treatment groups are compared using an analysis of variance (ANOVA), adjusted for the stratification of the study by primary diagnosis and a Mantel-Haenszel chi-square test for categorical variables. All tests were two-sided and assessed at a significance level of 5%.
For uncovered/malapposed struts, we opted for a Wilcoxon rank-sum test on the number or percentage of uncovered (malapposed) struts per patient. In addition, we performed a mixed logistic regression analysis including a random intercept to assess the individual probability of a strut being covered.
For the remaining parameters, we calculated summary statistics of these parameters per patient (mean, min and max) and again analysed these using a Wilcoxon rank-sum test. We also analysed the individual strut data for these, using a mixed model including a random intercept per patient. Since, in all cases, all these analyses yielded largely the same results, we opted to present the simplest analyses using the Wilcoxon rank-sum test.
The statistical analysis was performed using SAS/STAT software, Version 9.2 (TS2M3; SAS Institute, Cary, NC, USA).
Results
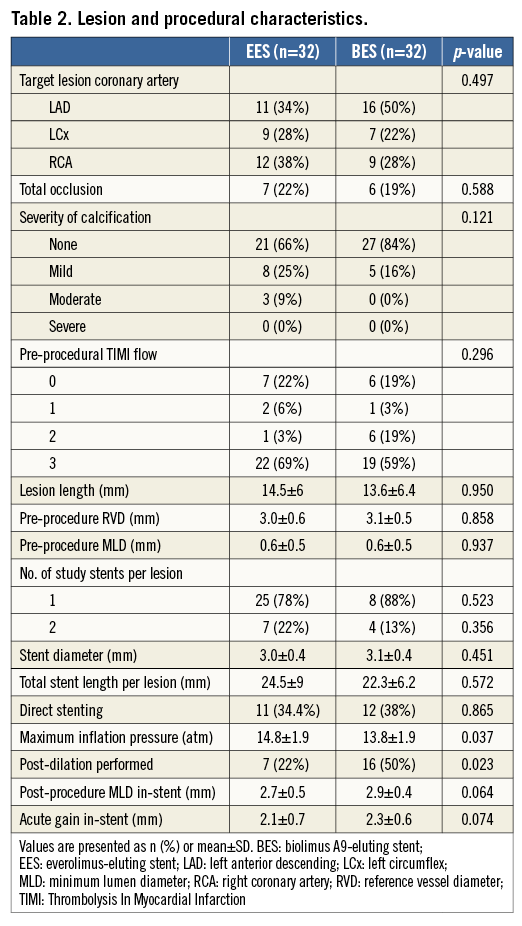
Between June 2009 and January 2011, 64 patients were enrolled in the trial: 21 patients presented with STEMI, 21 with NSTEMI and 22 had stable disease or marker-negative unstable angina. The study flow is presented in Figure 2. Baseline patient, lesion and procedural characteristics were largely comparable between groups and are presented in Table 1 and Table 2. The exception was a higher percentage of the use of post-dilation in the BES arm. PCI was successful in all patients. One patient in the BES group suffered acute vessel closure due to distal edge dissection immediately following the procedure and was treated with implantation of an additional stent.
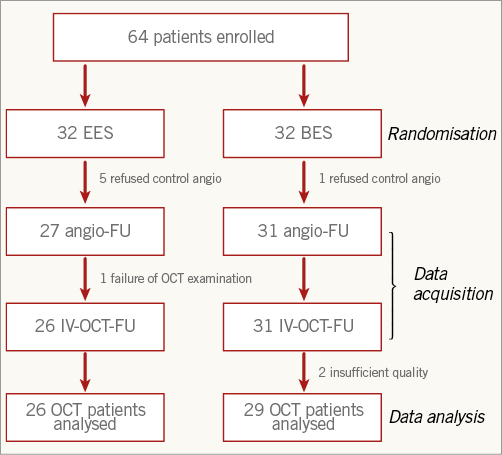
Figure 2. Study flow chart.
Fifty-eight patients underwent control angiography at nine months (six patients, five in the EES arm and one in the BES arm, refused control angiography). In one patient, the OCT examination could not be performed due to technical reasons. Two OCT examinations were of insufficient quality for quantitative analysis. The OCT analysis group thus consisted of 55 patients, 26 in the EES group and 29 in the BES group. Quantitative data are presented in Table 3 and Table 4.
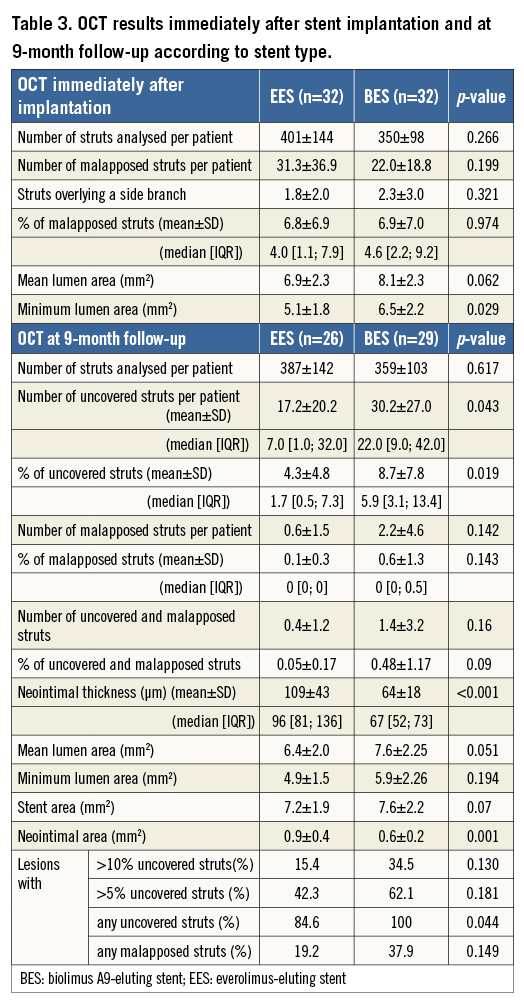
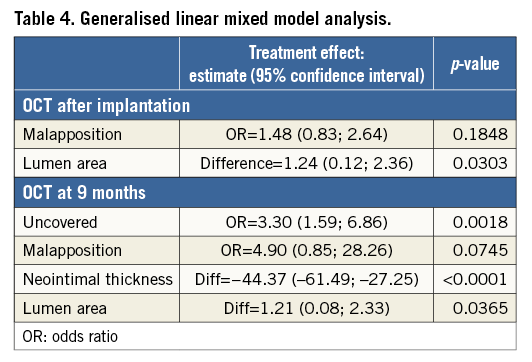
At follow-up, the percentage of uncovered struts was significantly lower in patients treated with EES as compared to those treated with BES (4.3% vs. 8.7%, p=0.019). Mean neointimal thickness was 109±43 µm in EES vs. 64±18 µm in BES (p<0.001). The percentage of malapposed struts immediately after implantation was similar in both groups (6.8% in EES vs. 6.9% in BES, p=0.974). At follow-up, the percentage of malapposed struts was low in both groups (0.1% vs. 0.6%, p=0.143).
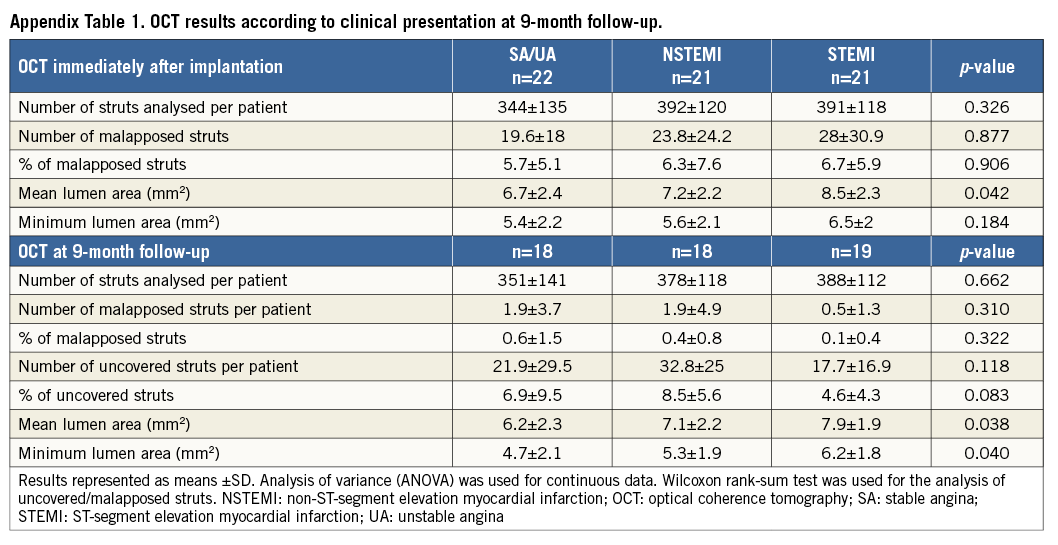
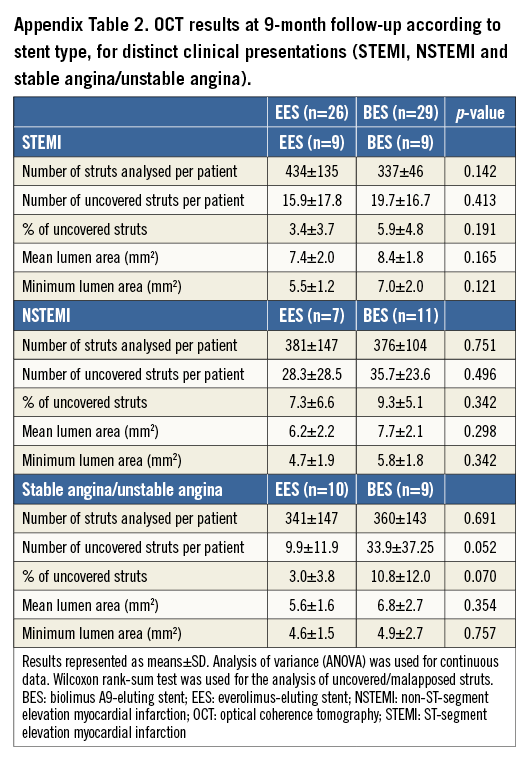
OCT results are reported according to categories of clinical presentation (Appendix Table 1). We did not observe statistically significant differences in the percentage of uncovered or malapposed struts. Appendix Table 2 shows the results of nine-month OCT parameters in EES vs. BES for the different clinical indications. Figure 3 is a graphical representation of the length of the stented segments and the burden of uncovered struts in both treatment arms. There were no major complications during the OCT examinations.
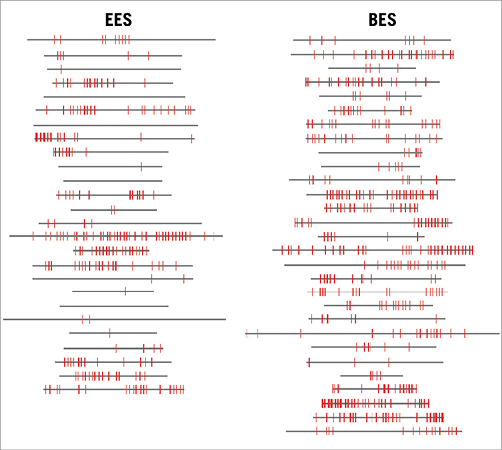
Figure 3. Graphical representation of stent coverage in lesions with EES versus BES. Grey horizontal bars represent stented segments. Uncovered struts are represented by red lines.
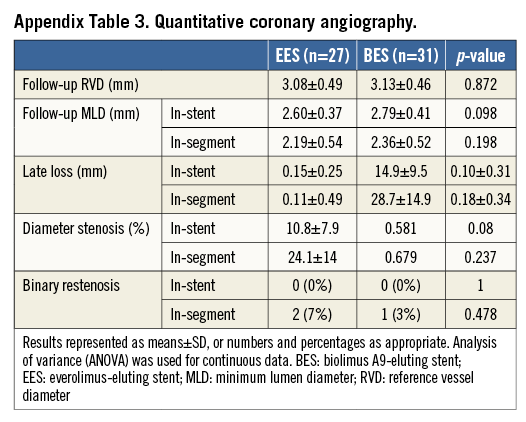
QCA results are presented in Appendix Table 3. There were no differences between groups regarding MLD, LLL and binary restenosis rate.
With respect to the clinical outcome, all patients were alive at one year. Except for the one patient in the BES arm who suffered acute vessel closure due to distal edge dissection necessitating repeat intervention and causing periprocedural myocardial infarction, there was no instance of ST or TLR in either group.
As to the duration of dual antiplatelet therapy, all patients in the EES group and all but two in the BES group were on a combination of acetylsalicylic acid and clopidogrel at 12 months. The two exceptions were a patient with atrial fibrillation on a combination of warfarin and clopidogrel and another patient in whom clopidogrel was stopped at 10 months after the index procedure due to a gastrointestinal bleeding.
Discussion
In the STACCATO study, we observed a significantly lower percentage of uncovered struts at nine-month follow-up in patients treated with EES as compared to BES in de novo coronary artery lesions, as assessed with OCT.
Differences in stent strut coverage at nine months may in part be explained by the stent platform, the polymers used to control drug release, and the antiproliferative drug itself.
First, the platform of the XIENCE stent has a thinner strut (89 µm, i.e., 81 μm metal plus 8 μm permanent polymer) configuration, compared to the 136 µm (125 μm metal plus 11 μm bioresorbable polymer) of the BioMatrix stent. Reduced strut thickness may result in less arterial injury during stent implantation, and may accelerate reendothelialisation owing to the lower physical height of the strut to be covered12,13. Second, the XIENCE EES utilises a permanent copolymer composed of vinylidene fluoride and hexafluoropropylene monomers, providing nearly complete everolimus release at 90 days7.
In contrast, the BioMatrix BES uses a biodegradable PDLLA poly (D3L-lactide) polymer, which is gradually degraded into carbon dioxide and water during six to nine months after implantation. This coating is limited to the abluminal stent surface, aiming at selective release of the drug to modulate exclusively the proliferation of smooth muscle cells in the media without interfering with the reendothelialisation process at the luminal side.
The attractive concept of polymer degradation, over time leaving only a bare metal stent in the coronary artery wall, potentially eliminates the problems of inflammatory reaction associated with first-generation DES. This has, however, been challenged by the observation that both parent polymer compounds as well as their degeneration products may cause inflammation14. These responses may have played a role in the delayed arterial healing associated with BES observed in the current study. The primary endpoint of our study may therefore have been influenced by such ongoing inflammatory reaction nine months after implantation of BES. However, serial OCT assessment of BES and sirolimus-eluting stents (SES) at nine and 24 months in the LEADERS trial did not show significant improvement in the percentage of uncovered struts in patients treated with BES, suggesting that healing with the BioMatrix stent remains virtually unchanged after nine months15. Finally, abluminal PLA polymer appears to be more susceptible to delamination and cracks during insertion and stent expansion, affecting polymer integrity, which could lead to suboptimal healing16.
A last aspect in which both devices differ is the drug loaded on the stent. Biolimus A9 is the limus analogue with the highest lipophilicity used for drug elution on currently available stents, improving drug uptake by the coronary vessel wall17. Higher local tissue drug concentrations may explain the numerically lower neointimal thickness and LLL as well as the higher MLD after BES in the current study. However, low LLL with BES, as consistently reported in previous studies3, may impact favourably on antirestenotic efficacy, while adversely affecting strut coverage and healing.
Overall, the results of our study confirm the favourable healing characteristics reported with EES18. It is more challenging to put into perspective the less favourable results of stent strut coverage in the BES arm of the current study. Based on pathology and OCT studies, there is substantial evidence that impaired healing contributes to the risk of stent thrombosis associated with DES2,19. Although our study was not powered to detect differences in clinical events, we did observe a difference in healing between BES and EES at nine months.
In a landmark meta-analysis of randomised controlled trials (RCT), including over 50,000 patients20, EES was associated with a lower risk of ST versus other non-biolimus-eluting stents, but also versus BMS. Similarly, the BioMatrix stent was non-inferior to first-generation SES in the LEADERS RCT, and showed a lower rate of ST with BES compared to SES at four years3,4. Recent data with the Nobori™ (Terumo Corp., Tokyo, Japan) stent, a biodegradable polymer BES almost identical to BioMatrix, showed clinical non-inferiority to EES in the COMPARE II trial21. Numerically, however, the rates of definitive ST at one year were slightly higher with BES (0.7% vs. 0.4% with EES). Similarly, in the SORT OUT V trial22, BES showed a slightly higher rate of definitive ST at one year as compared with SES (0.7% vs. 0.2%, respectively). Although difficult to compare, taken together, the data of these large randomised trials do not support the hypothesis of a lower rate of stent thrombosis and thus increased safety in BES over other second-generation DES. The results of the current STACCATO trial might in part explain why this lack of safety benefit with BES over EES is seen in the larger clinical trials. Furthermore, our results do not support a shorter duration of dual antiplatelet therapy in patients treated with biodegradable polymer stents.
Limitations
A few limitations concerning this study merit further discussion. It was a single-centre trial and there was no independent OCT core lab analysis. However, the OCT analysis for stent strut coverage was performed by investigators blinded to treatment allocation, using an automated software system9. In addition, strut coverage results must be interpreted with caution, since OCT does not have sufficient resolution to determine the composition of the material covering stent struts and cannot determine endothelial functionality. With respect to procedural characteristics, we observed a slight imbalance, with BES lesions yielding higher post-procedural lumen and stent diameters and areas. This difference is most probably caused by higher stent diameters achieved in the BES platform when deployed at the pressures delivered by the operators and the higher rates of post-dilation in the BES compared to the EES arm. Although these differences are probably not large enough to impact on the global findings of the trial, these observations should also be considered a limitation of the current study. Finally, our study was not powered to detect clinical differences between the two stents.
Conclusions
In conclusion, a significantly higher percentage of uncovered struts was detected with OCT in the biodegradable polymer-coated BES compared with the durable fluoropolymer-coated EES at nine-month follow-up in the setting of STEMI, non-STEMI and stable/unstable angina. Our findings do not support improved midterm healing characteristics of stents with biodegradable polymer-based biolimus elution as compared to current second-generation permanent polymer DES.
| Impact on daily practice Second-generation drug-eluting stents (DES) with biodegradable polymer coating have been proposed as the optimal solution for the problem of delayed coronary artery healing characteristics observed with first-generation permanent polymer-coated DES. However, in the current randomised STACCATO trial, comparing this type of device with permanent biocompatible polymer-coated second-generation DES, using the percentage of uncovered struts at long term assessed with optical coherence tomography as a surrogate endpoint, healing characteristics with biodegradable polymer DES were slightly worse. The findings of our study do not support a preferential use of stents with biodegradable polymer to reduce the risk for stent thrombosis. |
Funding
The research project funding was provided by Research Foundation Flanders (FWO – G.0690.09N), Brussels, Belgium, and the Industrial Research Foundation KU Leuven (IOF – ZKC2992), Leuven, Belgium. T. Adriaenssens is also supported by a Clinical Doctoral Grant of the FWO.
Conflict of interest statement
T. Adriaenssens, C. Dubois and W. Desmet received unrestricted research grants from Biosensors and Abbott Vascular. T. Adriaenssens is a consultant for St. Jude Medical. The other authors have no conflicts of interest to declare.
Supplementary data