Abstract
Aims: To our knowledge, no randomised study has compared rates of uncovered stent struts in everolimus (EES) vs. new-generation zotarolimus-eluting (ZES-R) stents in acute coronary syndrome (ACS). The aim of our study was to evaluate the completeness of neointimal coverage with optical coherence tomography (OCT) in ACS patients treated with drug-eluting stents (DES) comparing EES versus new-generation ZES-R.
Methods and results: All eligible ACS patients admitted to four Italian centres with a clinical indication for culprit lesion intervention were randomised 1:1 to EES or ZES-R. The primary study endpoint was the percentage of uncovered stent struts evaluated by optical coherence tomography (OCT) at six months. Secondary endpoints were the percentage of malapposed stent struts, percent neointimal hyperplasia cross-sectional area (CSA) and major adverse cardiac events (MACE) at six months. A total of 60 patients were randomised to EES (n=29) or ZES-R (n=31). No differences were observed in baseline characteristics between the two groups. Overall, 31.7% presented with STEMI, of which 68.4% were anterior. The other patients comprised 41.7% NSTEMI and 26.7% troponin-negative ACS. A mean of 1.3±0.6 lesions were treated per patient, with a mean of 1.3±0.5 stents per lesion. At 30 days there was one sudden death. Six-month OCT analysis was performed in 25 lesions in the EES group and in 24 lesions in the ZES-R group. There was no difference in the primary endpoint of uncovered stent struts between groups (EES 6.42% [3.27, 9.57] vs. ZES-R 7.07% [3.22, 10.92]; p=0.80). Furthermore, there were no differences between groups in the percentage of malapposed stent struts, either with (EES 1.19% [0.34, 2.04] vs. ZES-R 0.85% [0.40, 1.30]; p=0.49) or without coverage (EES 1.06% [0.12, 2.01] vs. ZES-R 0.24% [0.05, 0.44]; p=0.09). Percent neointima CSA was similar in both groups (EES 37.0% [18.6, 55.3] vs. ZES-R 26.6% [18.4, 34.8]; p=0.31). At six-month clinical follow-up, no additional patients died or suffered MI. There were four MACE in the EES group and one in the ZES-R group.
Conclusions: In our study, in patients presenting with ACS, both EES and ZES-R had low percentages of malapposed and uncovered stent struts at six-month OCT analysis.
Introduction
Some concerns have been raised regarding the risk of late and very late stent thrombosis (ST) following drug-eluting stent (DES) implantation1-4. Although premature discontinuation of dual antiplatelet therapy (DAPT) has been described as one of the most powerful predictors of ST2, it is thought that incomplete endothelialisation with delayed healing may be a causative factor5. New-generation DES have superior efficacy and potentially an improved safety profile as compared with first-generation DES6-8.
In the setting of acute coronary syndromes (ACS) where there is a thrombus-rich milieu, higher rates of ST have been reported9,10. Some of the proposed reasons for this are a higher occurrence of malapposition11 and incomplete neointimal stent strut coverage5,12,13.
The aim of this study, therefore, was to evaluate the completeness of neointimal coverage with optical coherence tomography (OCT) in ACS patients treated with DES comparing everolimus-eluting stents (EES) versus new-generation zotarolimus-eluting stents (ZES-R).
Methods
STUDY DESIGN
This study (NCT01239654) was a prospective, randomised, multicentre national trial funded by the Italian Ministry of Health. The APICE OCT study was part of a larger research project entitled “Acute Coronary Syndrome Activity of Platelets after Inhibition and Cardiovascular Events (APICE)”. The protocol was approved by the ethical committee of each participating centre (San Raffaele Scientific Institute, Milan, Italy; Careggi Hospital, Florence, Italy; Ospedale Civile, Mirano, Italy; and Universita Magna Graecia, Catanzaro, Italy).
The inclusion criteria were: ACS (defined as ST-elevation myocardial infarction [STEMI], non-ST-elevation myocardial infarction [NSTEMI], or ACS with negative troponin); the presence of one or more de novo stenoses equal to or greater than 70% in the native coronary arteries; and clinical indication for percutaneous coronary intervention (PCI) with new-generation DES. The exclusion criteria were: a culprit lesion in a venous or arterial graft, in-stent restenosis, significant unprotected left main coronary artery disease, reduced left ventricular ejection fraction (≤30%), chronic kidney disease (creatinine ≥1.5 mg/dL), contraindications to DAPT, or a concurrent medical condition with a life expectancy ≤12 months. Full written informed consent was obtained from all patients. Mediolanum Cardio Research (Milan, Italy) was responsible for the electronic data capture database and data management, as well as safety monitoring procedures. Cardiovascular Research Foundation (New York, NY, USA) was the independent OCT core laboratory for this study.
STUDY PROCEDURES
Following angiography and the identification of the culprit lesion, each patient was randomised through a computer-generated randomisation system, stratified by centre, with block size equal to four (1:1 allocation ratio) to EES (PROMUS Element™; Boston Scientific, Natick, MA, USA) or ZES-R (Endeavor Resolute; Medtronic Inc., Minneapolis, MN, USA) implantation. It was not mandatory for study protocol to perform an immediate post-procedure OCT examination.
Clinical follow-up was obtained at one and six months with a clinic visit or telephone call. By study protocol, angiographic follow-up with OCT was performed at six months using the C7-XR or M3 imaging system using non-occlusive flush techniques (St. Jude Medical, St. Paul, MN, USA) with a motorised pullback of 20 mm/s and rotation speed of 100 frames/s for C7-XR, and 3 mm/s and 20 frames/s for M3, respectively. OCT images were digitally stored and analysed using proprietary software (LightLab Imaging Inc., Westford, MA, USA) by the core laboratory which was blinded to the study stent types. OCT cross-sections were selected for quantitative analysis located every 1 mm throughout the entire length of each stent, and 5 mm-long segments proximal and distal to the stent edges, i.e., 1 mm apart. However, if a selected frame contained artefacts, the closest artefact-free frame was analysed instead. Stent and luminal cross-sectional areas (CSA) were measured at each still frame and the neointimal hyperplasia (NIH) area was calculated as the stent CSA minus the luminal CSA.
Each observed strut in each analysed still frame was classified as either completely or incompletely apposed to the vessel wall and according to whether there was complete tissue coverage or not. Each stent strut was classified into one of the following categories: (a) well apposed covered; (b) well apposed uncovered; (c) malapposed covered; (d) malapposed uncovered; (e) orifice branch site covered, or (f) orifice branch site uncovered (Figure 1). To assess malapposition, we measured the distance from the centre of the stent blooming artefact to the nearby endoluminal surface of the intima. These measurements were performed blinded to the stent type. After measurements had been made and entered into the database, the thickness of each stent strut was automatically subtracted from this distance. Malapposition was present if the measured distance was greater than the thickness of the stent metal plus the polymer: 88 µm for PROMUS Element (81 µm+7 µm) and 97 µm for Endeavor Resolute (91 µm+6 µm). An uncovered strut was defined as having no visible tissue on the luminal surface of the strut. If the strut was covered with tissue, the tissue thickness was measured from the endoluminal surface of the tissue to the centre of the blooming artefact of the strut. Qualitative imaging of every frame was also performed to detect the presence of abnormal intraluminal tissue (AIT). We report the OCT results per lesion assessed. In order to avoid misclassification of small image artefacts, only AIT >0.25 mm diameter were included14-16.
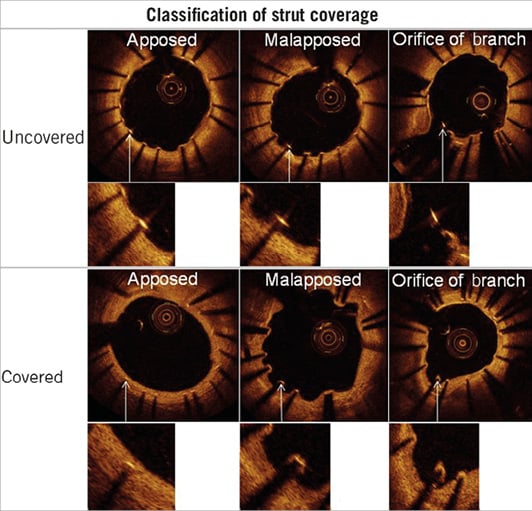
Figure 1. Stent strut classification. An illustration of the classification of stent strut apposition and coverage used in the study.
STUDY ENDPOINTS
The primary study endpoint was the percentage of uncovered (without tissue coverage) stent struts per lesion by OCT analysis at six months. Secondary OCT endpoints of the study were the percentage of malapposed stent struts and the percent NIH CSA. The secondary clinical endpoint was the occurrence of major adverse cardiac events (MACE), defined as cardiac death, myocardial infarction (MI), target vessel revascularisation (TVR) and ST during hospitalisation and at one and six months.
SAMPLE SIZE
No evidence about the expected magnitude of the effect was available when the trial was designed, as the rate of uncovered struts in the study stents in the setting of ACS has never been specifically evaluated. Therefore, no formal sample size calculation based on the primary endpoint could be done. However, based on prior clinical studies17,18 using other DES, we expected a sample size of 60 patients would be sufficient for our exploratory study.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±SD and analysed with the Student’s t-test or Wilcoxon rank-sum test depending on the variable distribution. Categorical variables are presented as numbers and percentages and were compared with the chi-squared test with Yates correction for continuity or the Fisher’s exact test as appropriate. For strut or lesion level analysis, a model with generalised estimating equation (GEE) approach was used to compensate for any potential cluster effect of multiple struts in the same lesion or multiple lesions in the same patient. The GEE model was developed by use of a working correlation structure of compound symmetry and a two-level nested cluster effect for patient and lesion within patient. The results are presented as least squares means with 95% confidence intervals (CI). Statistical analysis was performed with Statistical Package for Social Sciences version 18.0 (SPSS Inc., Chicago, IL, USA) or SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). A p-value of <0.05 was considered statistically significant.
Results
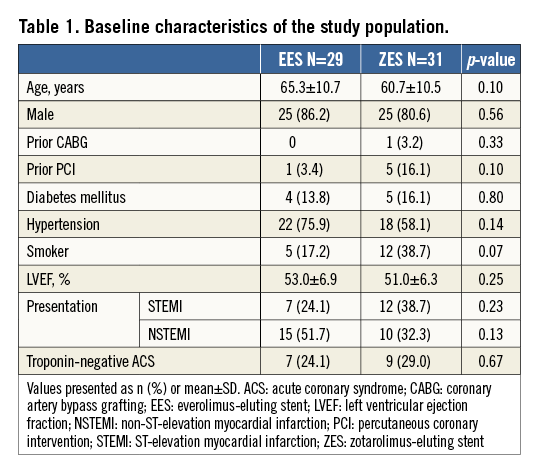
From September 2010 to February 2011, a total of 60 patients were enrolled: 29 were randomised to EES and 31 to ZES-R. The majority were male (83.3%) with a mean age of 63.0±10.8 years. Overall, 31.7% presented with STEMI, of which 68.4% were in the anterior territory. The other patients comprised 41.7% with NSTEMI and 26.7% with troponin-negative ACS. There were no significant differences between the study groups in baseline clinical characteristics, as illustrated in Table 1.
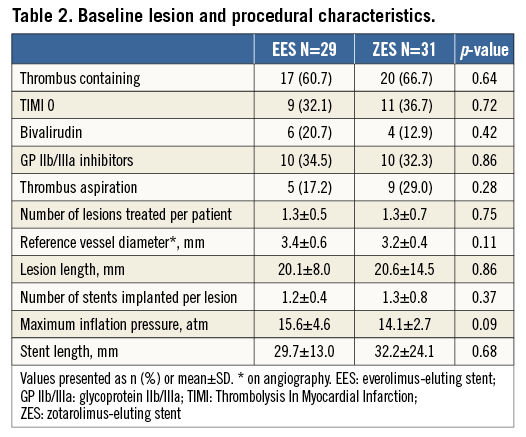
With regard to lesion and procedural characteristics, no difference was observed between the two groups (Table 2). Notably, a high proportion of lesions had angiographic evidence of thrombus (61.7%). Glycoprotein IIb/IIIa inhibitors were used in 33.3% and bivalirudin in 16.6% of the patients. Only 23.3% of cases had manual thrombus aspiration. A mean of 1.3±0.6 lesions were treated per patient, with a mean lesion length of 20.3±11.6 mm. Each lesion had a mean of 1.3±0.5 stents implanted at a maximum of 14.8±3.8 atmospheres. The mean total stent length used in each patient showed no difference between stent types (EES 29.7±13.0 mm vs. ZES-R 32.2±24.1 mm; p=0.683).
PRIMARY STUDY ENDPOINT
Forty-two patients (with 25 lesions in the EES group and 24 lesions in the ZES-R group) underwent OCT as per protocol at six months. In most (n=38) of the cases the C7-XR OCT system was used; the M3 OCT system was used in only four cases. In total, there were 6,490 analysable struts in the EES and 7,246 analysable struts in the ZES-R groups. There was no difference in the primary endpoint of uncovered stent struts between stent groups (EES 6.42% [3.27, 9.57] vs. ZES-R 7.07% [3.22, 10.92]; p=0.80). The overall number of covered struts in the EES group was 6,136, and 6,675 in the ZES-R group.
Of note, when we analysed separately patients with STEMI (14 lesions) vs. those with NSTEMI/ACS (35 lesions), there remained no difference in the percentage of uncovered stent struts, respectively 8.56% [3.29, 12.82] vs. 5.96% [3.35, 8.58]; p=0.38).
SECONDARY OCT ENDPOINTS
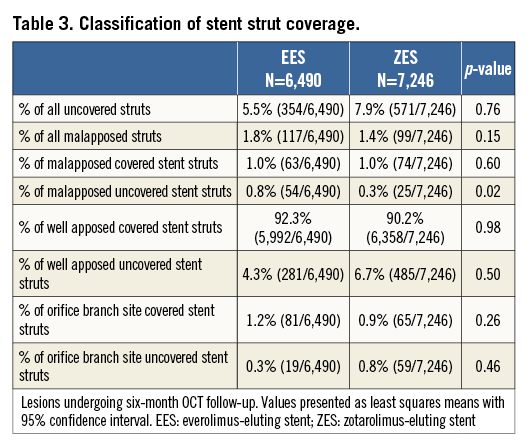
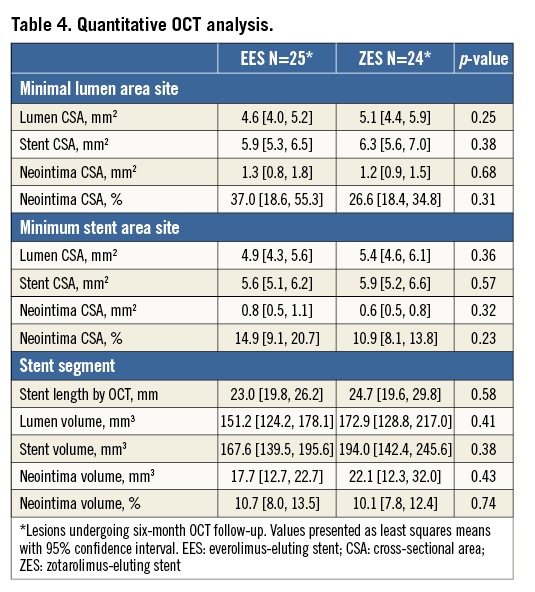
Stent apposition and coverage classifications as assessed by OCT are shown in Table 3 and Figure 1. In total, 117 EES struts and 99 ZES-R struts showed evidence of malapposition. There was no statistically significant difference between groups in the percentage of malapposed stent struts overall (EES 2.27% [0.61, 3.93] vs. ZES-R 1.10% [0.52, 1.68]; p=0.19), neither was there any difference in those with coverage (EES 1.19% [0.34, 2.04] vs. ZES-R 0.85% [0.40, 1.30]; p=0.49) or without coverage (EES 1.06% [0.12, 2.01] vs. ZES-R 0.24% [0.05, 0.44]; p=0.09). Furthermore, there was no difference in the neointimal thickness (EES 102 µm [82, 121] vs. ZES-R 87 µm [70, 104]; p=0.27). With regard to the percentage NIH area at the minimal lumen area site, the percentage neointima CSA was 37.0% (18.6, 55.3) in EES and 26.6% (18.4, 34.8) in ZES-R (p=0.31). There was no difference in percentage neointimal volume (EES 10.7% [8.0, 13.5] vs. ZES-R 10.1% [7.8, 12.4]; p=0.74). Further quantitative OCT analysis is demonstrated in Table 4.
The presence of AIT was noted in four lesions. Three cases of AIT (two EES and one ZES-R) occurred at the proximal stent edge of a covered strut. One case of AIT was noted within the body of the stent at the site of a small side branch with an overlying malapposed EES stent strut. There were no cases of plaque rupture behind the stent noted in our study population.
Furthermore, on analysis of STEMI vs. NSTEMI/ACS individually, there remained no differences other than a higher rate of orifice side branch covered stent struts in the STEMI population (1.87% [0.64, 3.10] vs. 0.69% [0.41, 0.96]; p=0.07).
SECONDARY CLINICAL ENDPOINTS
During the index hospital admission, no adverse events were reported. At 30-day follow-up, one sudden death had occurred at eight days in the EES group which was adjudicated to be a probable ST. No other MACE were observed within this time period.
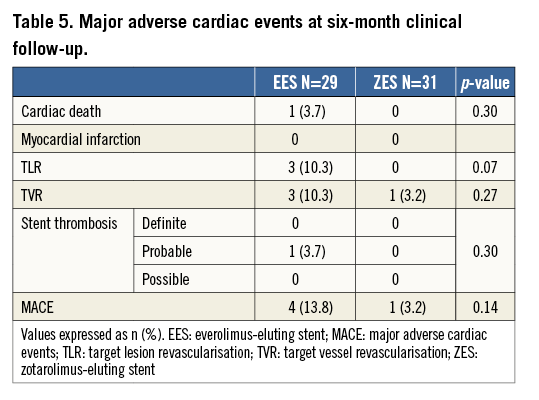
At six-month clinical follow-up, there were no additional deaths or ST. No recurrent MIs were reported (Table 5). Three (10.3%) patients in the EES vs. none in the ZES-R group had a target lesion revascularisation (TLR) (p=0.07). One patient in the ZES-R group had a TVR. No difference in MACE was observed between the two study groups (EES 13.8% vs. ZES-R 3.2%; p=0.14).
Among the four cases of TLR, two had no evidence of neointima formation by OCT evaluation. The first case appeared to be a stent deformation (Figure 2A, Figure 2B). The second case had evidence of a partially deformed stent strut in the left anterior descending artery which angiographically appeared as “hazy” just proximal to the side branch. A third case had a crushed stent with red cell-rich thrombus in the RCA (Figure 3A-Figure 3C).
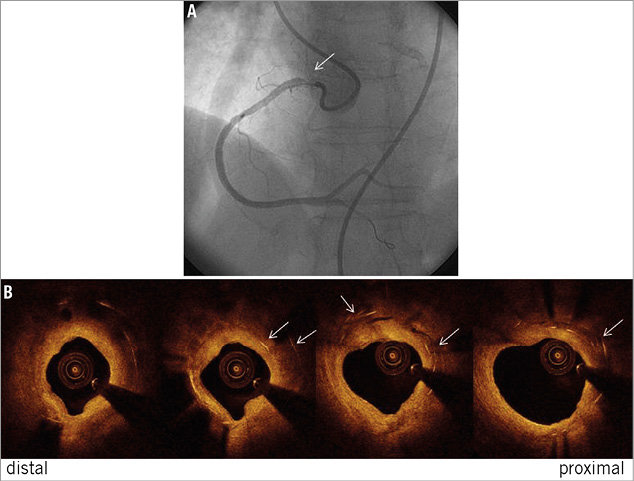
Figure 2. Angiographic (A) and OCT (B) images showing a stent deformation from a case of TLR occurring at the ostium of a right coronary artery (see arrow). White arrows demonstrate double layers of struts within one stent indicating stent deformation.
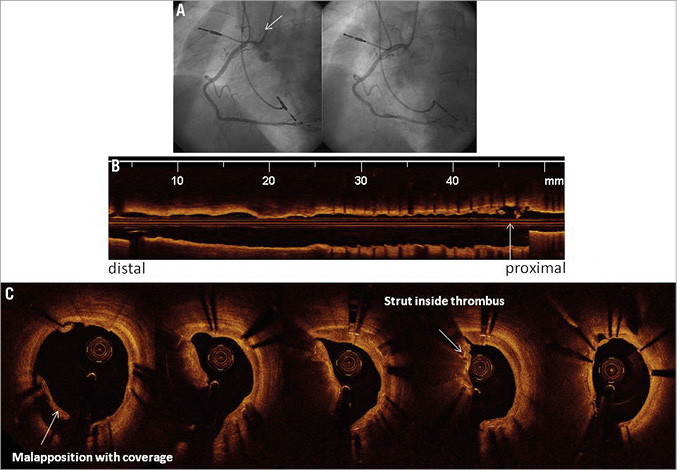
Figure 3. Angiographic baseline and post-intervention images (A) and OCT images (B & C) showing a focal crushed stent causing, at the ostium of the coronary artery, a lumen narrowing that was treated with target lesion revascularisation (TLR). No neointima proliferation was observed inside the stent. In panel B, the longitudinal OCT image demonstrates the site of TLR close to the proximal end of the stent (arrow). In panel C, cross-sectional OCT images showed red cell-rich thrombus within the crushed stent.
Discussion
The main findings of our study were as follows: 1) both EES and ZES-R were associated with an extremely low percentage of uncovered and/or malapposed stent struts, even in the setting of ACS; 2) there were no differences noted between EES and ZES-R in the occurrence of uncovered and/or malapposed stent struts; 3) no significant clinical differences were observed between EES and ZES-R at six-month follow-up.
To our knowledge, this is the first study to assess by OCT neointimal coverage in patients with ACS following new-generation DES implantation. These newer stents have been demonstrated in clinical studies to have improved efficacy and safety profiles in comparison to the first generation of DES6,7,19.
Some OCT studies have been conducted with new-generation DES in the setting of chronic stable angina showing a low occurrence (1.6%) of uncovered stent struts following EES implantation20. Most of the OCT studies were conducted in mixed populations21-24, with one such study evaluating EES (60% ACS) reporting an overall percentage of uncovered struts of 4.4±4.7% at nine months with a trend towards a higher percentage of uncovered struts in patients with ACS vs. stable angina (9.8±12.1% vs. 5.3±7.4%; p=0.058)23. Furthermore, in the RESOLUTE All Comers trial there was a subgroup of patients (n=58) who underwent OCT analysis at 13 months. No differences were observed between stent types in the number of uncovered stent struts with the new-generation ZES-R stent (ZES-R 7.4% vs. EES 5.8%; p=0.378)24. Recently, a comparable neointimal thickness and low percentage of uncovered stent struts with EES (3.3%) vs. ZES-R (3.4%) has been reported at nine-month OCT analysis in a population consisting of half ACS patients25. It must be noted that, in the prior reported EES studies, XIENCE (Abbott Vascular, Santa Clara, CA, USA) stents were used. These are similar to the PROMUS Element EES stents (Boston Scientific) used in our study with regard to the same drug and polymer; however, the PROMUS Element has a platinum chromium platform, which may have some differences from the cobalt chromium platform of the XIENCE stent in terms of radial strength and recoil.
In our study, the population was composed entirely of patients with ACS (31.7% STEMI; 41.7% NSTEMI; 26.7% troponin-negative ACS) who were randomised to EES vs. ZES-R, and the primary endpoint was the percentage of uncovered stent struts by OCT. At six months, uncovered stent struts were relatively infrequent and there was no difference between the study stents (EES 6.42% [3.27, 9.57] vs. ZES-R 7.07% [3.22, 10.92], p=0.80). Our result is comparable with that observed in the OCT substudy of the “Harmonizing Outcomes with Revascularisation and Stents in Acute Myocardial Infarction” (HORIZONS-AMI) trial analysing first-generation DES in the AMI setting15. In HORIZONS-AMI, 5.7±7.0% of the paclitaxel-eluting stent struts were uncovered at 13 months. Interestingly, an extremely low percentage of uncovered stent struts (0.3%) was reported at nine months by Kim et al with the first ZES stent available (Endeavor; Medtronic Inc.), 55% of which had an ACS22. Of note, the initial ZES stent used in this study comprised a phosphorylcholine polymer, which has been shown to act similarly to bare metal stents, with the current ZES-R comprising the BioLinx polymer (Medtronic) which is made of three different polymers to enable a finer and more sustained drug elution. The different DES generations used and the different duration of follow-up clearly limit the comparison of these reports.
One of the reasons for more uncovered struts in an ACS population might be the higher occurrence of malapposition because of a large thrombus burden. Incomplete stent apposition and delayed coverage have been shown to be more frequent in DES implanted during STEMI26. Moreover, a study has shown that DES implantation into a ruptured plaque with a necrotic core may impair stent strut endothelialisation, increasing the risk of subsequent ST27. Recently, Guagliumi et al have demonstrated with OCT that patients with late ST following DES implantation had a higher percentage of uncovered and malapposed stent struts compared with control subjects21. In our study, 61.7% were thrombus-containing lesions by angiography; however, only 1.06% (0.12, 2.01) of the EES and 0.24% (0.05, 0.44) of the ZES-R struts were malapposed without neointimal coverage. This is similar to that demonstrated in the HORIZONS-AMI study15. Furthermore, we observed a very low occurrence of AIT in our study, which is reassuring in the setting of ACS.
Our encouraging results with the new-generation stents in this study may be due to the fact that these stents offer more uniform suppression of neointimal proliferation. In a study by Miyoshi et al, only 85.8% of struts of SES at six months were well apposed with neointimal coverage28, lower than the 92.3% demonstrated in our study. Furthermore, the original ZES were shown to have the lowest rate of both uncovered and malapposed struts in a comparison study with PES and SES17, with almost complete neointimal coverage at nine-month follow-up22.
In our study, the six-month MACE rate (8.3%) was extremely encouraging and mostly driven by TLR rates. It is interesting to note that two of the TLR events actually had no neointima formation on OCT follow-up and indeed represented deformed or crushed stents. As OCT analysis was not performed immediately post procedure, these appearances may have been present from the outset and not noticed at the index procedure.
Study limitations
This study analysed a biological effect (stent strut coverage) using a novel technology, namely OCT. The impact of such an effect on clinical outcome has still to be defined. In addition, our study was not powered for the secondary clinical endpoints. The lack of immediate post-procedural OCT images precludes determining the incidence of acute stent malapposition, as well as changes over time. OCT images selected for analysis were 1 mm apart and not closer (as has been done in some other studies). It is not clear whether this will introduce variability; however, in the current study, both groups were analysed in the same way.
Conclusions
Both EES and ZES-R have low levels of malapposed and uncovered stent struts at six-month OCT analysis in patients presenting with ACS. However, further studies are required to determine the relationship between OCT findings and clinical outcomes in the longer term.
| Impact on daily practice Stent thrombosis (ST), although a rare event, remains a severe complication after stent implantation owing to its high morbidity and mortality. ST occurs more frequently in certain complex patient subsets, such as patients presenting with acute coronary syndromes (ACS). The high thrombus burden present in such patients may, in fact, lead to less stent coverage and more malapposition of stent struts, which are advocated factors possibly related to ST. Hence, this study, which shows a high stent coverage with new-generation DES in the setting of ACS, adds confidence that the use of these devices in such high-risk patients is safe and effective. |
Funding
This study (NCT01239654) was funded by the Italian Ministry of Health.
Conflict of interest statement
A. Maehara has received a research grant from and is a consultant for Boston Scientific. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Moving OCT image demonstrating stent deformation, observed at the ostium of a right coronary artery, from a case of target lesion revascularisation (see also Figure 2).
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Moving OCT image demonstrating stent deformation, observed at the ostium of a right coronary artery, from a case of target lesion revascularisation (see also Figure 2).

