Abstract
Aims: The aim of this study was to compare the efficacy and safety of zotarolimus-eluting stents (ZES), sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI).
Methods and results: This study was a prospective, single-blind, multicentre, randomised trial. The primary endpoint was major adverse cardiac events (MACE) at 12 months post-procedure, defined as cardiac death, recurrent myocardial infarction (MI), or ischaemia-driven target lesion revascularisation (TLR). An angiographic substudy was performed at nine months among 348 patients. From October 2006 to April 2008, 611 patients with STEMI undergoing primary PCI were randomly assigned to treatment with ZES (n=205), SES (n=204), or PES (n=202). The cumulative incidence of MACE was 5.9% in the ZES group, 3.4% in the SES group and 5.7% in the PES group at 12-month follow-up (p=0.457). There was a trend towards a lower rate of ischaemia-driven TLR at 12- (p=0.092) and 18-month (p=0.080) follow-up in the SES group compared to the ZES and PES groups. No difference was observed in rates of cardiac death, recurrent MI and combined death and/or recurrent MI among three groups at 12- and 18-month follow-up. The rate of stent thrombosis was similar among the three groups (2.0% in each group, p=1.000).
Conclusions: As compared with SES and PES, the use of ZES in patients with STEMI undergoing primary PCI, showed similar rates of MACE, cardiac death and recurrent MI at 12 and 18 months. There was a trend towards a higher rate of TLR with ZES or PES compared to SES.
Introduction
Although several registry studies have shown conflicting results1-4, randomised controlled trials describing the use of drug-eluting stents (DES) during primary percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI) have demonstrated that DES are superior compared to bare metal stents (BMS) in the reduction of major adverse cardiac events (MACE)5-8. In previous randomised trials and registry studies conducted in patients with stable coronary artery disease, late luminal loss, rate of angiographic restenosis or target lesion revascularisation (TLR) varied according to the type of DES9-12. To date, however, there is limited data comparing the use of different types of DES in patients with STEMI. More-over, recent concerns have emerged on the safety of DES including risk of stent thrombosis (ST) that might be even more pronounced among STEMI patients13-16. For these reasons, we evaluated the clinical and angiographic outcomes of a series of consecutive patients with STEMI treated with zotarolimus-eluting stents (ZES, Endeavor; Medtronic, Minneapolis, MN, USA), sirolimus-eluting stents (SES, Cypher; Johnson & Johnson, Miami Lakes, FL, USA) and paclitaxel-eluting stents (PES, Taxus Express2; Boston Scientific, Natick, MA, USA) as part of a primary PCI strategy.
Methods
Study design and patient population
This prospective, single-blind, multicentre, randomised study called the Korean Multicentre Endeavor (KOMER) acute myocardial infarction (AMI) trial, was conducted to evaluate the efficacy and safety of ZES, SES and PES in consecutive patients undergoing primary PCI in STEMI from October 2006 to April 2008. The study protocol was approved by the ethics committee at each participating institution. All patients gave written informed consent before enrolment. We enrolled consecutive patients who were 18 years of age or older if they had an AMI with ST-segment elevation (≥ 20 minutes of chest pain and at least 2 mm of ST-segment elevation in at least two contiguous leads or a new left bundle-branch block). Reperfusion was expected to be achieved within 12 hours after the onset of symptoms, and the native coronary artery was considered to be suitable for primary PCI with stent implantation (diameter stenosis >50%, ≥2.5 mm to ≤4.0 mm, de novo lesion in native coronary artery). We excluded patients if they had received thrombolytic therapy; if the infarction was caused by in-stent thrombosis or restenosis; if there was a known hypersensitivity or contraindication to aspirin, clopidogrel, heparin, zotarolimus, sirolimus, paclitaxel, stainless steel or contrast media; if they were participating in another clinical trial; if cardiogenic shock was evident before randomisation; if the left ventricular ejection fraction was below 25%; if they had left main stenosis (>50% by visual estimate); if they had severe kidney dysfunction (creatinine level >3.0 mg/dL or dependence on dialysis); or if the estimated life expectancy was less than 12 months.
Selection and randomisation
Randomisation was performed either immediately after coronary angiography if the infarct-related vessel was spontaneously patent or after re-establishing coronary-artery blood flow by the placement of a guidewire or by balloon angioplasty. Random assignments to the treatment groups were generated in blocks of four and were distributed in random tables to each participating centre. Patients were randomly assigned to the groups in a 1:1:1 ratio. Patients received either a ZES, SES or PES. Patients, but not investigators, were unaware of the treatment assignment.
Procedure
Patients were pre-medicated with aspirin (at least 100 mg) and unfractionated heparin (10,000 IU). A loading dose of 300 mg of clopidogrel was administered either before or immediately after PCI. A glycoprotein IIb/IIIa receptor blocker was administered at the discretion of the operator. Coronary angiography was performed through the femoral or radial artery with standard techniques.
The use of thrombectomy devices, intravascular ultrasound, distal protection devices and an intra-aortic balloon pump was at the operators’ discretion. Direct implantation of a stent without previous balloon angioplasty was allowed if the culprit lesion was adequately visualised during the initial contrast injection or after guidewire placement. In case of insufficient stent expansion, the stent was dilated after placement with another angioplasty balloon that was shorter than the total length of the stent. If more than one stent was implanted, the same type of stent was recommended. Intervention in non-infarct-related arteries during the initial procedure was discouraged. Heparin was administered throughout the procedure in order to maintain an activated clotting time of 250 seconds or longer. Final angiography was performed to obtain views similar to those obtained before the procedure.
Data collection and follow-up
Clinical follow-up was performed at 30 days, 6, 12 and 18 months after the procedure. Combined antiplatelet therapy included daily administration of aspirin (100 mg) and clopidogrel (75 mg). Dual antiplatelet therapy was recommended for at least 12 months, and aspirin therapy was recommended indefinitely. Patients were unaware of their treatment assignments throughout the follow up period.
Quantitative coronary angiography
Technicians at an independent angiographic core laboratory (Severance Hospital, Seoul, Republic of Korea), who were unaware of treatment assignment, analysed all angiographic images using an off-line QCA system (CMS, Medis Medical Imaging System, Nuenen, The Netherlands). Binary restenosis was defined as stenosis of more than 50% of the luminal diameter. Late luminal loss was calculated as the difference between the minimum luminal diameter immediately after the procedure and at nine months. In-segment was defined as the in-stent segment plus the proximal and distal 5 mm edge segments. Flow in the infarct-related vessel was graded according to the Thrombolysis in Myocardial Infarction (TIMI) trial classification.
Study endpoint and definitions
Procedural success was defined as no laboratory deaths, no emergency bypass surgery, and TIMI 3 flow in the distal part of the infarct-related artery with a residual stenosis of less than 30%. Cardiac death included death from AMI, cardiac perforation or pericardial tamponade; an arrhythmia or conduction abnormality; complications of the interventional procedure at baseline; stroke (including bleeding) within 30 days after the procedure or in connection with the procedure. All deaths were considered to have been from cardiac causes unless a non-cardiac cause could be identified. Recurrent MI was defined as recurrence of clinical symptoms or occurrence of electrocardiographic changes accompanied by a new increase of creatine kinase-MB to >3 times the upper limit of normal. The level of creatine kinase required for the diagnosis of recurrent MI depended on the interval from the index infarction: the creatine kinase level had to be at least 1.5 times the previous value if new symptoms appeared within 48 hours and at least three times the upper limit of normal if new symptoms appeared after 48 hours. Ischaemia-driven TLR was defined as repeated PCI or bypass grafting of the target lesion, driven by clinical symptoms of myocardial ischaemia, a positive stress test, or electrocardiographic evidence of ischaemic changes at rest attributable to the target lesion.
The primary endpoint of the study was MACE, defined as the composite of cardiac death, recurrent MI, or ischaemia-driven TLR at 12 months. The secondary endpoints included MACE at 18 months; individual components of the composite primary endpoint at 12 and 18 months; the rate of procedural success, stent thrombosis and late luminal loss in both in-stent and in-segment at nine-month angiographic follow-up.
Definition of stent thrombosis
Stent thrombosis was classified by the Academic Research Consortium definition as definite (when presence of an acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion), probable (when unexplained death within 30 days after the procedure or acute MI involving the target vessel territory without angiographic confirmation), acute (when <24 hours after procedure), subacute (when one day to 30 days after procedure), or late (when >30 days after procedure).
Statistical analysis
All analyses were performed according to the intention-to-treat principle. Baseline data are presented as proportions or mean (±standard deviation [SD]) values. The differences among the DES groups were evaluated by analysis of variance or the nonparametric Kruskal-Wallis test for continuous variables, if appropriate. Fisher’s exact test was used for the analysis of categorical variables. The cumulative incidence rates of the primary and secondary endpoints during the 18-month follow-up period were analysed by the Kaplan–Meier method. The significance of differences in rates of the endpoints between treatment groups was assessed by the log-rank test. All statistical analyses were performed with SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). A two-sided p-value of less than 0.05 was considered to indicate statistical significance.
Results
Patient population
From October 2006 to April 2008, 611 patients with STEMI undergoing primary PCI were randomly assigned to treatment with ZES (n=205), SES (n=204) or PES (n=202). A trial flow diagram is shown in Figure 1. The baseline features of the groups were well matched (Table 1). The mean age was 59.8 years; 78.9% of the patients were men; and the prevalence of diabetes mellitus was 20.8%.
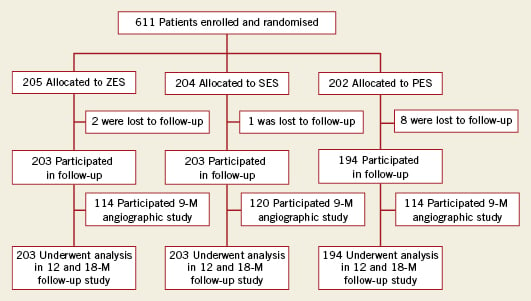
Figure 1. KOMER AMI study design diagram.
Procedural results
The angiographic characteristics (Table 2) and procedural results (Table 3) were also similar among the groups except that the lesions in the patients assigned to receive PES were slightly larger, necessitating the use of slightly larger stents compared to the other two groups. Almost 50% of patients had single vessel disease and underwent PCI of the left anterior descending artery.
Twenty percent of patients received glycoprotein IIb/IIIa inhibitors. The sizes of infarcts, reflected by the mean peak value of the creatine kinase MB isoenzyme, were similar among the three groups. Procedural success was achieved in 92% to 94% in each group.
Clinical outcomes at 30-day follow-up
Follow-up data at 30 days after PCI were available for all patients (Table 4). There were no differences in the occurrence of cardiac death (four deaths in ZES patients due to: heart failure [n=2], sudden death [n=1], recurrent MI [n=1]; two deaths in SES patients due to: heart failure [n=1], sudden death [n=1]; one death in PES patients due to cardiac rupture), recurrent MI and combined cardiac death and/or recurrent MI. No ischaemia-driven TLR events occurred in the first 30 days post procedure. The cumulative incidence of MACE at 30 days was 2.4% in the ZES group, 2.9% in the SES group and 2.0% in the PES group (p=0.82). The occurrence of ST events was not statistically different among the three groups.
We documented four (2.0%) ST in the ZES group including one acute ST; four (2.0%) subacute ST in the SES group; and one acute and two subacute (2.0% total) in the PES group. ARC definite ST events occurred in three ZES, four SES, and four PES patients, and ARC probable ST events occurred in one ZES patient.
Clinical outcome at 12- and 18-month follow-up
Clinical follow-up was completed in 99.0% of patients in the ZES group, 99.5% in the SES group and 96.0% of those in the PES group. The cumulative incidence of MACE was 5.9% in the ZES group, 3.4% in the SES group and 5.7% in the PES group at 12-month follow-up (Table 4). No difference was observed in terms of cardiac death, recurrent MI and combined death and/or recurrent MI among the three groups at 12- and 18-month follow-up (Table 4). As compared to SES, ZES showed a trend towards increased ischaemia-driven TLR at 12- and 18-month follow-up, but this was not statistically significant (Table 4 and Figure 2). The cumulative incidence of MACE was 6.9% in the ZES group, 3.9% in the SES group and 6.2% in the PES group at 18-month follow-up (Table 4 and Figure 2). Late ST occurred in one patient (0.5%) in the PES group and none in the ZES or SES groups (p=NS).
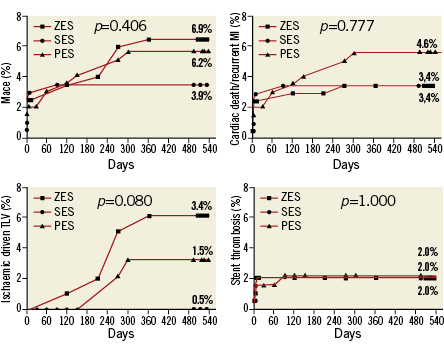
Figure 2. Kaplan–Meier time-to-event curves for MACE, cardiac death/ recurrent MI, ischaemia-driven TLR and stent thrombosis. ZES: zotarolimus-eluting stent; SES: sirolimus-eluting stent; PES: paclitaxel-eluting stent; MACE: major adverse cardiac event; MI: myocardial infarction; TLR: target lesion revascularisation
Angiographic follow-up study
Analyses of the angiographic follow-up data at nine months included 114 ZES patients, 120 SES patients and 114 PES patients. Minimal luminal diameters (MLD) were 2.04±0.70 mm in the ZES group, 2.59±0.47 mm in the SES group and 2.43±0.63 mm in the PES group. In-stent late luminal loss was higher in the ZES group (0.68±0.67 mm) than in the SES group (0.11±0.40 mm, p<0.001) or the PES group (0.37±0.57 mm, p<0.001, Table 3, Figure 3); and compared to the SES group, in-stent late luminal loss in the PES group was higher (p=0.002, Figure 3). In-segment late luminal loss was also higher in the ZES group than the SES or PES groups (Figure 3). The rate of in-segment binary restenosis in the ZES group was higher at 14.0% compared with 1.7% in the SES group (p<0.001) or 4.4% in the PES group (p=0.020, Table 3).
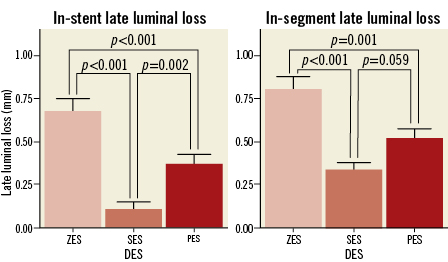
Figure 3. In-stent and segment late luminal loss at nine-month follow-up angiography. ZES: zotarolimus-eluting stent; SES: sirolimus-eluting stent; PES: paclitaxel-eluting stent; DES: drug-eluting stent
Discussion
The main results of our study conducted to compare the benefits and safety of ZES, SES and PES in patients with STEMI undergoing primary PCI are as follows:
First, the cumulative incidence of MACE (a composite of cardiac death, recurrent MI or ischaemia-driven TLR) and the rate of ST were not significantly different among the three DES groups at 12- and 18-month follow-up. No difference was observed in rates of cardiac death, recurrent MI and combined death and/or recurrent MI among the three groups at 12- and 18-month follow-up.
Second, although the rates of cardiac death or recurrent MI were similar in comparison with previous randomised trials5,6,17, the rate of TLR was relatively low, which resulted in a lower rate of MACE.
Third, the angiographic follow-up study showed lower rates of in-stent late luminal loss and restenosis in the SES group, which translated into a trend towards a lower ischaemia-driven TLR in the SES group compared to the ZES and PES group.
Although several randomised trials have shown that DES are associated with a significant reduction in restenosis and TLR in patients with STEMI undergoing primary PCI5,6,18,19, data comparing clinical and angiographic outcomes among different DES in these patients is limited. A meta-analysis of previous randomised trials showed that SES and PES compared to BMS are associated with a significant reduction in TLR at one and two-year follow-up in STEMI patients undergoing primary PCI20,21. The benefits from SES in terms of reducing clinical and angiographic restenosis without an increase in death or MI have been confirmed in subsequent randomised trials such as the TYPHOON5 and SESAMI trials7. However, PES showed only a weak trend toward a reduction in TLR (5.3% vs. 7.8%, p=NS) compared to BMS. These results might be associated with the less marked reduction of neointimal hyperplasia with paclitaxel compared to sirolimus, or a better outcome with the control stent17. In contrast to the PASSION trial, PES in the HORIZON-AMI trial6 showed significantly reduced angiographic evidence of restenosis and recurrent ischaemia necessitating repeat revascularisation procedures compared to BMS. In the PASEO trial8, as compared to BMS (14.4%), both PES (4.4%, p=0.023) and SES (3.3%, p=0.016) were associated with a significant reduction in TLR at one-year follow-up. As a result, PES and SES were associated with significant benefits in MACE (PES: 16.7%, p=0.015; SES: 15.6%, p=0.009, respectively) compared to BMS (32.2%) at two-year follow-up. In comparison with previous randomised trials, our study showed lower rates of TLR but similar rates of cardiac death or recurrent MI.
There are a number of possible explanations for this difference. First, ischaemia-driven TLR was performed for only those who had clinical symptoms of myocardial ischaemia, a positive stress test or electrocardiographic evidence of ischaemic changes at rest attributable to the target lesion. In post-MI patients, even significant restenosis may have developed in the absence of ischaemic symptoms, owing either to partial viable myocardium or to a defective warning system. In fact, one total occlusion of the stent at nine-month follow-up angiography and two subacute ST at second staged PCI during hospitalisation were noted without clinical presentation in the ZES group. Second, the study was performed in patients with a short (19.5 mm) culprit lesion where there was a decreased risk of restenosis (1.7% in SES, 4.4% in PES). Third, our study design did not include routine angiographic follow-up in all patients, and restenosis observed during routine follow-up angiography could have led to reintervention without symptoms or objective evidence of ischaemia, thus increasing the event rate.
Recent concerns have emerged regarding an increased risk of ST associated with any DES13-16. Compared to stable coronary artery disease, in the highly thrombotic environment at the time of stent implantation, the potential for suboptimal stent deployment and decreased blood flow in a vessel that supplies infarcted myocardium might be pronounced in the setting of primary PCI for STEMI. As most episodes of ST result in MI, this increase with DES may impact mortality, particularly after primary PCI, as reinfarction is a major determinant of survival. In a recent prospective multicentre primary angioplasty registry (PREMIER), the use of DES was associated with a higher risk of mortality within the first six months, but most of the events were related to early discontinuation of antiplatelet therapy22. However, the results of two large, randomised, controlled trials, TYPHOON and PASSION, suggest that DES can be used safely in the setting of primary PCI with an acceptable risk of ST5,17. Furthermore, long-term follow-up of the TYPHOON and STRATEGY studies demonstrated no increase in sustained safety and efficacy4,18. The overall rate of ST (2.0%) in our study was compatible with other studies5,6,17 and without statistically significant differences among the three groups. Like other studies, most of the ST events were acute and subacute, which might be associated with the highly thrombotic environment in the AMI setting rather than DES type. Only one late ST occurred at three months after PCI in the PES group, which was associated with discontinuation of clopidogrel for dental care.
Recently, the ZEST-AMI trial showed similar comparative results among the three DES (Endeavor stents vs. Cypher stents vs. TAXUS Liberté stents) in a relatively smaller AMI population23. At 12 months, the cumulative incidence rates of MACE in the ZES, SES and PES groups were 11.3%, 8.2% and 8.2%, respectively (p=0.834), which were relatively higher than those of our study. Of interest, there was a non-significant trend in favour of ZES in the rate of ST compared to SES and PES (0% vs. 3.6% vs. 2.7%, p=0.157). However, this study did not have sufficient statistical power to allow definite conclusions23.
There are several potential limitations of our study. First, we made a serious error in sample size calculation. The revised power calculation requires a total of 1,623 patients, but we only recruited 611. Therefore, this study was underpowered to compare the efficacy and safety among DES in patients with STEMI. Second, the follow-up duration may be too short to conclude on the long-term efficacy and safety of DES in AMI. Third, our results cannot be generalised to all patients with AMI since high-risk patients were excluded, and due to a relatively late randomisation strategy (after initial angiography). Fourth, because small vessels (less than 2.5 mm) were excluded, our patient population had a relatively large (2.96 mm) mean reference diameter compared to those included in previous randomised trials. Fifth, to avoid any bias due to duration of clopidogrel prescription, we preferred to prescribe clopidogrel up to 12 months in all DES groups. Lastly, the rate of patients lost at follow-up was somewhat higher in patients in the PES group, which might affect the results.
Sources of funding
This work was supported by unrestricted grants from Medtronic, Inc.
Conflict of interest statement
The authors have no conflict of interest to declare.

