Abstract
Aims: We sought to investigate the impact of the self-apposing, sirolimus-eluting STENTYS stent on midterm and long-term stent apposition and strut coverage compared with a zotarolimus-eluting balloon-expandable stent in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PPCI).
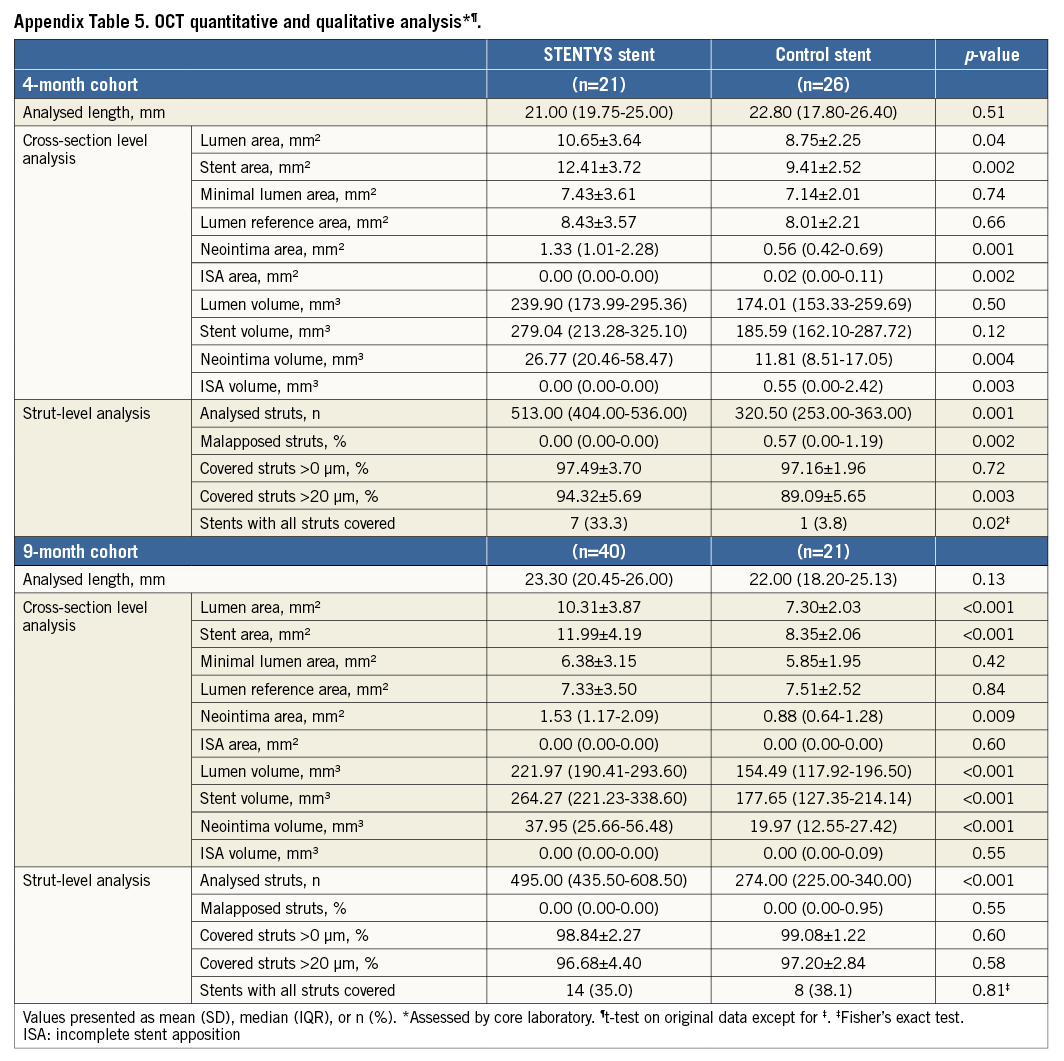
Methods and results: In the APPOSITION IV trial, 152 STEMI patients were randomised (3:2) to the self-apposing, sirolimus-eluting STENTYS stent or a commercially available zotarolimus-eluting balloon-expandable stent at 12 sites in five countries with angiographic follow-up and optical coherence tomography at four or nine months. At four months, a lower percentage of malapposed stent struts was observed in the STENTYS group (N=21; Nstruts=501) compared with controls (N=26; Nstruts=326; 0.07% vs. 1.16%; p=0.002) with significantly more covered struts, using a 20 µm cut-off (94.32% vs. 89.09%; p=0.003). At nine months, the primary endpoint (percentage malapposed stent struts) was similar in both groups (STENTYS, N=40; Nstruts=566; control, N=21; Nstruts=292), showing complete apposition (p=0.55) and near total (>96%) coverage (p=0.58).
Conclusions: In STEMI patients undergoing PPCI, the self-apposing, sirolimus-eluting STENTYS stent was equivalent to a conventional drug-eluting balloon-expandable stent with respect to late stent strut apposition and coverage at nine months. However, stent strut apposition and coverage at four months were significantly better in the STENTYS group.
Abbreviations
BMS: bare metal stent
DES: drug-eluting stent
MACE: major adverse cardiac events
OCT: optical coherence tomography
PPCI: primary percutaneous coronary intervention
QCA: quantitative coronary angiography
ST: stent thrombosis
STEMI: ST-segment elevation myocardial infarction
Introduction
Primary percutaneous coronary intervention (PPCI) with balloon-expandable stents remains the preferred reperfusion modality in patients with ST-segment elevation myocardial infarction (STEMI)1. Nevertheless, thrombus burden and vasoconstriction during acute STEMI can obscure the actual size of the infarct-related artery, paving the way for the implantation of undersized stents. This difficulty may, in turn, contribute to early or late malapposition, restenosis or stent thrombosis (ST)2, negating the beneficial effects of PPCI.
The STENTYS Self-Apposing® coronary stent system (STENTYS S.A., Paris, France) is designed to enable the stent to tailor its size to the vessel diameter when the actual size of the vessel is obscured and to provide full apposition as any remaining thrombus dissolves over time. Indeed, the expansive property of the STENTYS bare metal stent (BMS) was substantiated in the APPOSITION I trial, as evidenced by a 19% increase in stent area following a 19% increase in lumen area of the distal reference vessel at three days post STEMI, as measured by intravascular ultrasound3. Results from the randomised APPOSITION II trial extended these observations by demonstrating a tenfold reduction in stent strut malapposition with the self-apposing stent compared with conventional balloon-expandable BMS, as measured by optical coherence tomography (OCT) at three days4. Nonetheless, late stent expansion of the STENTYS BMS and vessel growth in APPOSITION I were offset by intimal proliferation with a 0.71 mm late loss at six months, indicating the necessity of a drug-eluting variant3. Moreover, whether improved early stent apposition in conjunction with local drug elution translates into sustained strut coverage at midterm and long-term follow-up after STEMI remains unknown.
Accordingly, we performed a prospective, multicentre, randomised trial to determine the impact of a sirolimus-eluting variant of the self-apposing STENTYS stent on strut apposition, strut coverage and late loss at four- or nine-month follow-up compared with the zotarolimus-eluting balloon-expandable stent in STEMI patients undergoing PPCI.
Methods
STUDY DESIGN AND POPULATION
The APPOSITION IV (Randomised comparison between the STENTYS Self-Apposing Sirolimus-eluting coronary stent and a balloon-expandable stent in acute myocardial infarction) was a prospective, randomised, international, multicentre trial. The study complied with the Declaration of Helsinki. The protocol was approved by the ethics committee at each participating centre, and informed written consent was obtained from all participating patients. The study organisation is listed in Appendix 1.
Patients 18 years of age and older presenting with symptoms consistent with STEMI lasting ≤12 hrs in duration, with ≥2 mm of ST-segment elevation in ≥2 contiguous leads, intended for PPCI were eligible for enrolment. Clinical exclusion criteria were: cardiogenic shock; left ventricular ejection fraction <30%; known hypersensitivity or contraindication to study medications, stainless steel, sirolimus, zotarolimus, or contrast material; coronary or cardiac intervention or major surgery of any kind ≤30 days before procedure; cerebrovascular accident or transient ischaemic attack <6 months previously. Angiographic eligibility required a de novo lesion ≤25 mm in length after achievement of reflow and reference vessel diameter (RVD) >2.5 to <4.0 mm by visual estimation. Exclusion criteria were: target vessel supplied by bypass vessel; myocardial infarction due to ST, or infarct lesion at a previously stented coronary artery; unprotected left main coronary disease with >30% stenosis by visual assessment; excessive tortuosity or calcification of the target vessel; two-vessel disease if additional procedures were anticipated ≤30 days; three-vessel disease with significant lesions in all three major epicardial vessels; aneurysmal dilation proximal or distal to the lesion.
RANDOMISATION AND TREATMENT
Before cardiac catheterisation, patients were administered aspirin 250-500 mg intravenously, 5,000 IU heparin and either 600 mg clopidogrel, 60 mg prasugrel, or 180 mg ticagrelor. Emergent coronary angiography was then performed. If all angiographic eligibility criteria were met, the patient was then randomised 3:2 to either the STENTYS Self-Apposing sirolimus-eluting stent (SES) or the balloon-expandable zotarolimus-eluting stent (Resolute™; Medtronic, Minneapolis, MN, USA) using a computer-generated randomisation process. A second randomisation (1:2 in the STENTYS arm; 1:1 in the control arm) was then performed for a four- or nine-month angiographic follow-up with OCT. The local principal investigator and research coordinators were aware of the study assignments.
All patients received procedural anticoagulation according to practice guideline recommendations5 and local standards of care. The use of glycoprotein IIb/IIIa inhibitors was left to operator discretion. Manual thrombus aspiration was recommended, whereas the decision to perform predilation was left to operator discretion. Side branch access could be created if the diameter was >2.25 mm with Thrombolysis In Myocardial Infarction (TIMI) flow grade <3, and/or stenosis >50%, and/or dissection >grade B. Side branches were to be treated with a provisional technique, since the STENTYS stent design with interconnectors allows this strategy. If the deployed STENTYS stents seemed underexpanded at any point along the culprit lesion with respect to RVD, post-dilation was mandatory until full expansion was obtained, whereas post-dilation in the control arm was left to operator discretion. Standard pharmacological therapies after the procedure included aspirin, P2Y12 inhibitors, beta-blockers, lipid-lowering agents, and angiotensin-converting enzyme inhibitors or angiotensin-II receptor blockers, according to current guidelines5.
INVESTIGATIONAL DEVICE
The STENTYS coronary stent is a self-apposing, nitinol, sirolimus-eluting stent (1.4 µg/mm² of stent) with a nominal strut width of 68 µm (0.0027”) incorporated in a proprietary coating ProTeqtor® (Hemoteq AG, Würselen, Germany), a durable polymer matrix of polysulphone and a soluble polyvinylpyrrolidone that acts as an excipient. The stent is compatible with a 6 Fr guide catheter and is delivered using a rapid exchange delivery system over a conventional 0.014” guidewire. The device is deployed by withdrawal of a retractable sheath and is available in three lengths (17, 22 and 27 mm) with diameters suitable for vessels ranging from 2.5-3.0 mm (small), 3.0-3.5 mm (medium), and 3.5-4.5 mm (large).
QUANTITATIVE CORONARY ANGIOGRAPHY AND OPTICAL COHERENCE TOMOGRAPHY
The detailed quantitative coronary angiography (QCA) and OCT methodologies have been previously reported4 and are briefly described in Appendix 2. All QCA and OCT images were analysed off-line by an independent core laboratory (Cardialysis, Rotterdam, The Netherlands).
ENDPOINTS AND FOLLOW-UP
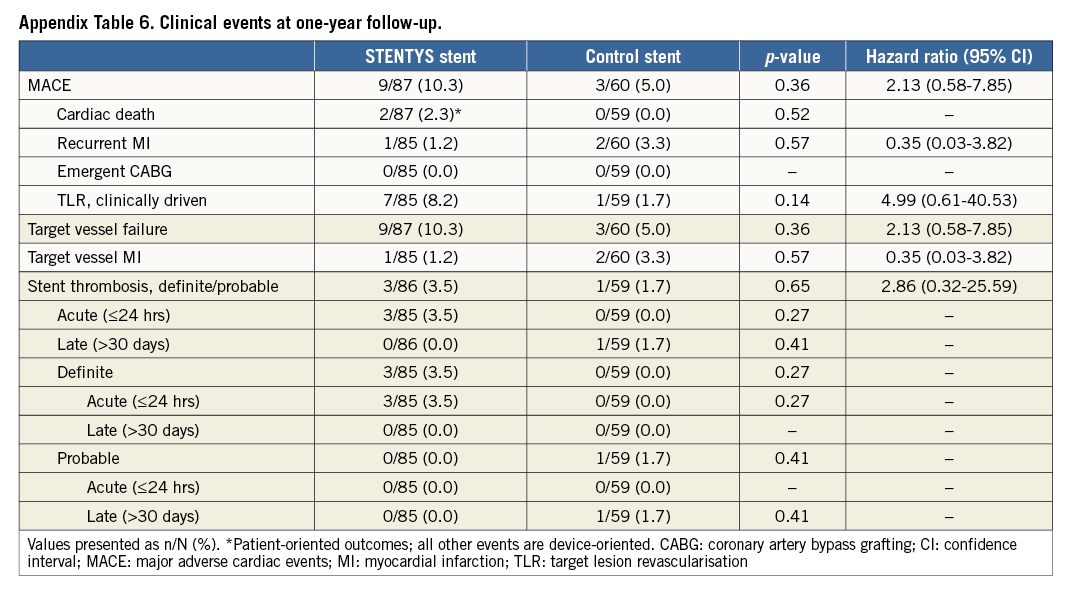
The primary endpoint was the percentage of malapposed stent struts at nine months by OCT assessment. Secondary endpoints consisted of OCT (four-month % malapposed struts; four-/nine-month % uncovered struts; four-/nine-month mean lumen/stent/ISA area and volume), angiographic (four-/nine-month in-stent/in-segment MLD and LLL; post-procedural TIMI flow grade, corrected TIMI frame count, TIMI myocardial perfusion grade), and electrocardiographic (ST-segment resolution 90 min post procedure) components. Major adverse cardiac events (MACE) (the composite of cardiac death, recurrent myocardial infarction [MI], emergent coronary artery bypass surgery, or clinically driven percutaneous or surgical target lesion revascularisation [TLR]), target vessel failure (the composite of cardiac death, target vessel MI [Q- or non-Q-wave], or clinically driven percutaneous or surgical target vessel revascularisation) and ST as per the ARC criteria6 comprised the clinical endpoints.
Angiographic assessment was performed after stent implantation and at four or nine months, while OCT was performed only at the randomised follow-up period. Clinical data were collected before, during and after the procedure, at discharge, at 30 days, and at four- or nine-month follow-up. All patients were followed up to one year.
POWER AND STATISTICAL ANALYSIS
With 90 assessable patients (N=60, STENTYS; N=30, controls), the trial had 90% power to detect a 0.095 ratio in the group proportions, as has been found in APPOSITION II4. This was based on 5% and 0.48% malapposed struts in the control and STENTYS groups, respectively, a multi-level analysis, and an intraclass correlation coefficient of 0.025, with a two-sided α=0.05. This power calculation accounted for a 10% drop-out rate.
The primary analysis was performed in the as-treated population. In this population, the follow-up windows were defined as 4±2 months and 9±3 months in the four- and nine-month cohorts, respectively. Baseline clinical characteristics were analysed on a patient level, whereas OCT data were analysed on per-patient, per-lesion, per-segment, and per-strut levels. Continuous variables are presented as mean (SD) or median (interquartile range) and were compared with the unpaired t-test or Mann-Whitney U test. Categorical variables are presented as numbers (%) and were compared with the chi-square or Fisher’s exact test. For segmental and strut analyses, continuous variables were compared using generalised estimating equations with exchangeable correlation to account for the clustering of values within each lesion and subject. Hazard ratios and 95% confidence intervals were estimated with a Cox proportional hazards model, with treatment as the only covariate. Computations were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA), with statistical significance set at p<0.05.
Results
PATIENTS AND PROCEDURES
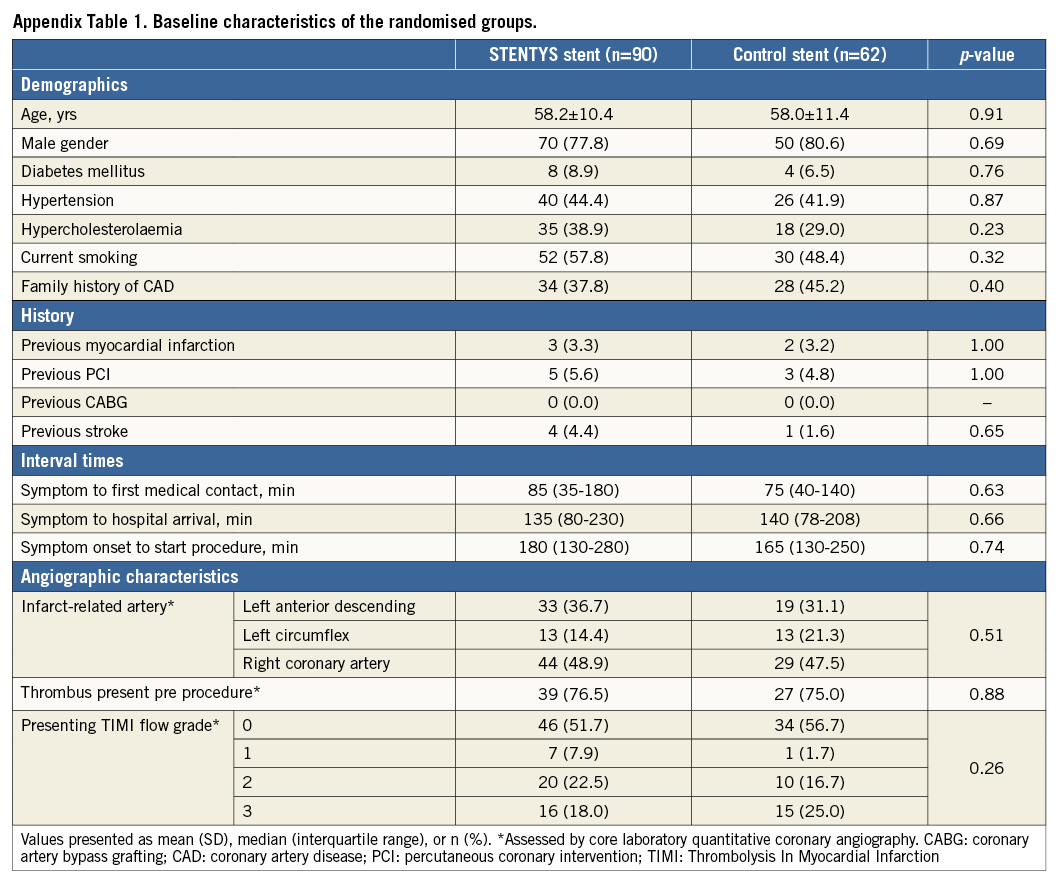
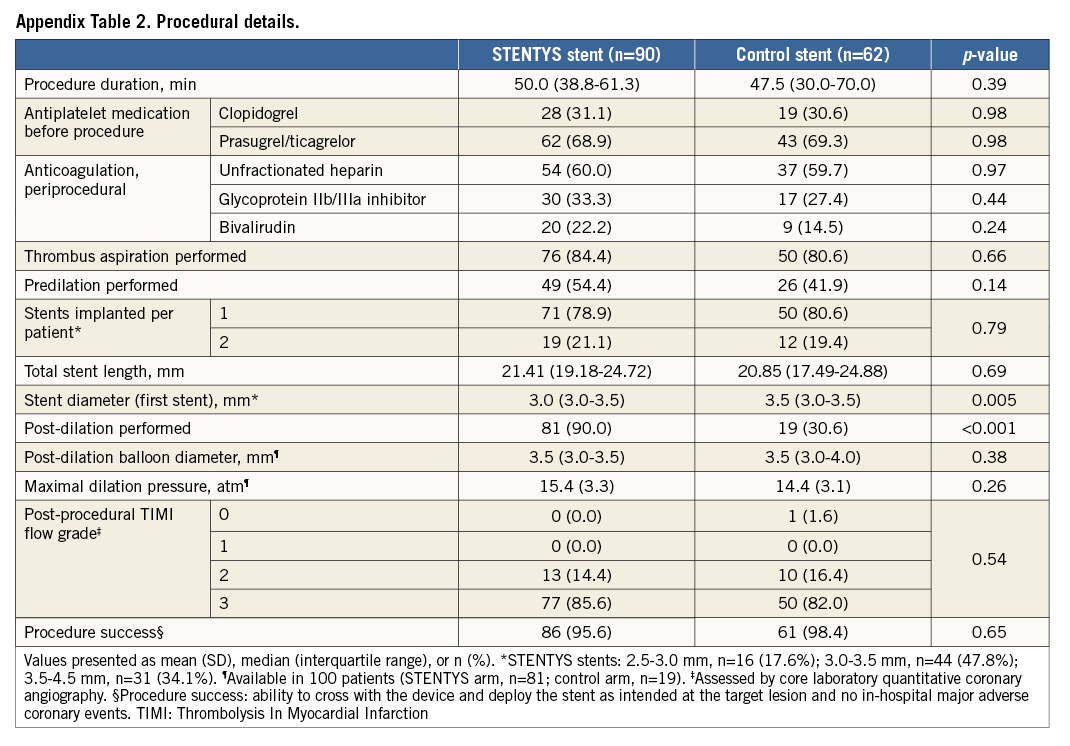
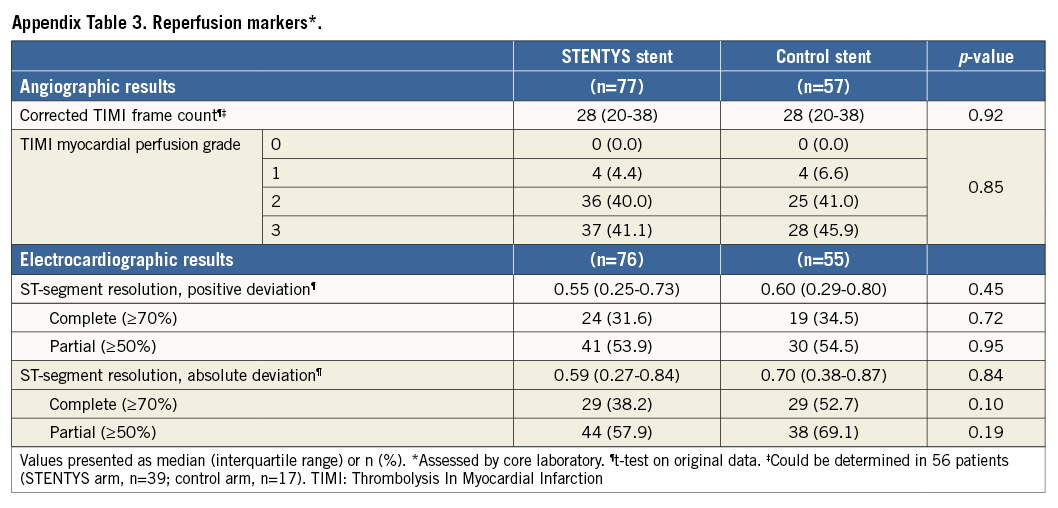
Between May 2012 and March 2013, 152 patients with STEMI were enrolled and randomised at 12 sites in five countries (Appendix 3) to the STENTYS stent (N=90) or a control stent (N=62) (Figure 1). Baseline clinical and angiographic features were well matched between the two groups (Appendix Table 1, Appendix Table 2). The mean age was 58 years, and 21% of patients were female. The infarct-related artery was the left anterior descending coronary artery in 34% of patients, the left circumflex coronary artery in 17%, and the right coronary artery in 48%. Aspiration was performed in approximately 83% of patients in each group. Post-dilation was performed in 90% of cases in the STENTYS group compared with 31% in the control group (p<0.001), with the mean maximal pressure and balloon diameter being similar in both groups. Procedure success was similar between groups (p=0.65) with no differences in angiographic or electrocardiographic markers of reperfusion (Appendix Table 3).
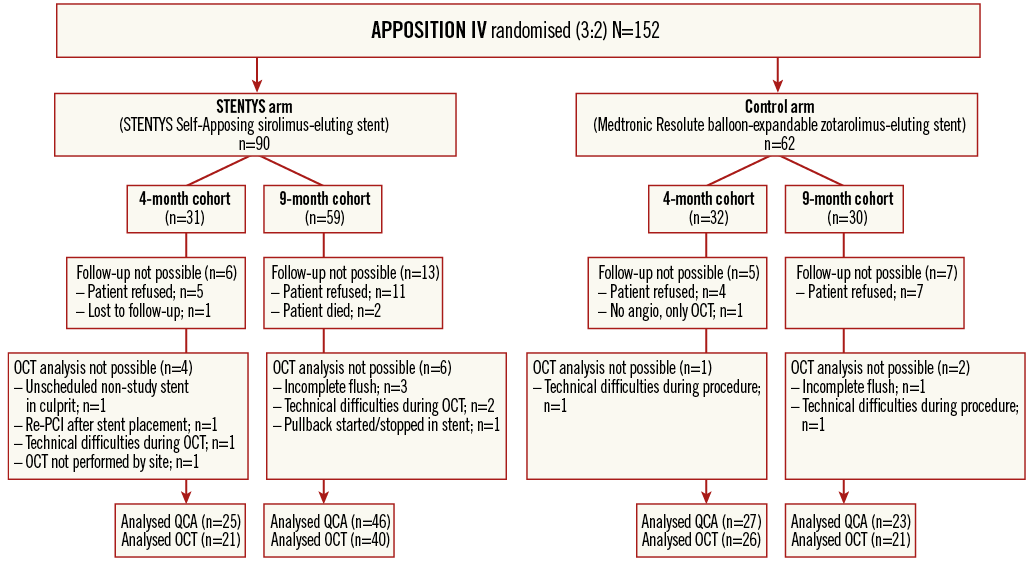
Figure 1. Study flow diagram. OCT: optical coherence tomography; PCI: percutaneous coronary intervention; QCA: quantitative coronary angiography
QUANTITATIVE CORONARY ANGIOGRAPHY
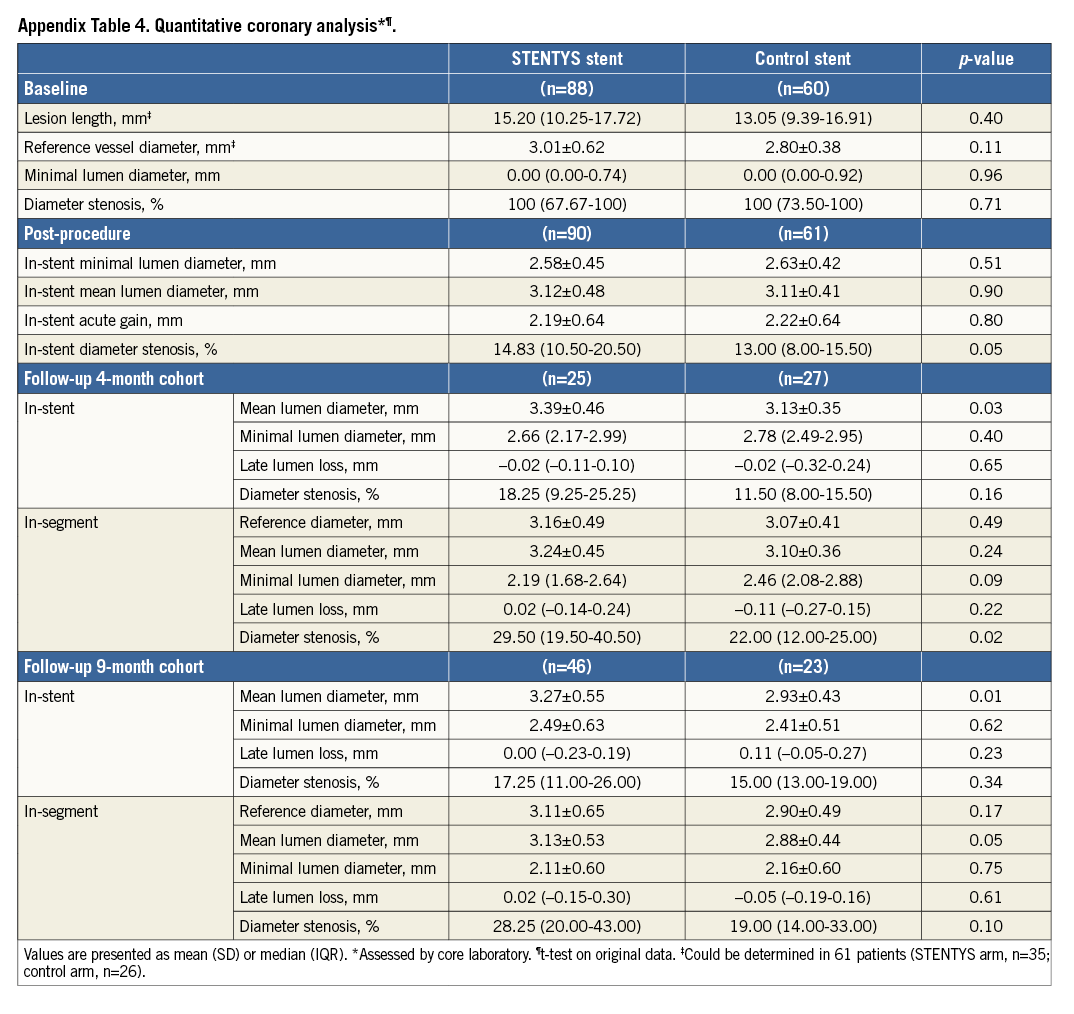
Baseline and post-procedure angiographic measurements were similar for the STENTYS and control groups (Appendix Table 4). At four months, the STENTYS stent had a larger in-stent mean lumen diameter than the control stent (3.39±0.46 vs. 3.13±0.35 mm; p=0.03), but LLL was equivalent (p=0.65). At nine months, the in-stent mean lumen diameter was 3.27±0.55 mm in the STENTYS group and 2.93±0.43 mm in the control group (p=0.01). Median LLL was 0.00 mm in the STENTYS group compared to 0.11 mm in controls without attaining statistical significance (p=0.23) (Appendix Table 4).
OPTICAL COHERENCE TOMOGRAPHY
Quantitative and qualitative OCT analyses are shown in Appendix Table 5. In the four-month cohort, a lower percentage of malapposed stent struts was observed in the STENTYS group compared with the control group (0.07±0.26% vs. 1.16±1.59%; p=0.002) (Figure 2). Mean ISA area in the STENTYS group was smaller compared with controls (0.00±0.01 vs. 0.07±0.10 mm²; p=0.002). With a cut-off of 20 µm, the STENTYS stent had significantly more covered struts than the control stent (94.32 vs. 89.09%; p=0.003). On a per-patient basis, 33.3% of stents in the STENTYS group were fully covered versus 3.8% in the control group (p=0.02) (Figure 3). Both mean lumen as well as mean neointima area was larger in the STENTYS group compared with controls (Figure 4). In the nine-month cohort, no differences were observed in strut apposition and coverage between groups (Figure 2, Figure 3, Appendix Table 5). Similar to the four-month cohort, mean lumen and neointima areas were larger in the STENTYS group at nine months (Figure 4). Stent apposition in the four- and nine-month cohorts is illustrated in Figure 5.
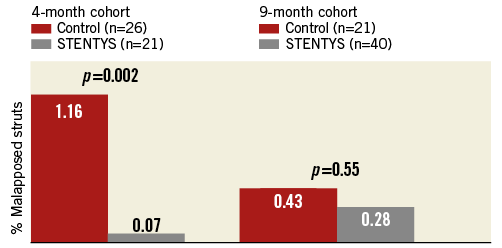
Figure 2. Strut malapposition. Optical coherence tomography-derived stent strut malapposition at four- or nine-month follow-up.
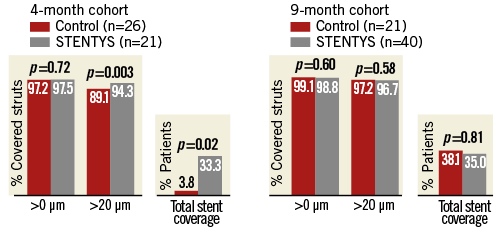
Figure 3. Strut coverage. Optical coherence tomography-derived stent strut coverage (>0 µm, >20 µm, or total strut coverage) at four- or nine-month follow-up.
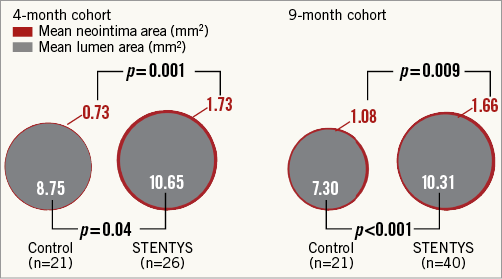
Figure 4. Cross-sectional areas. Optical coherence tomography-derived mean lumen and mean neointima areas at four- or nine-month follow-up.
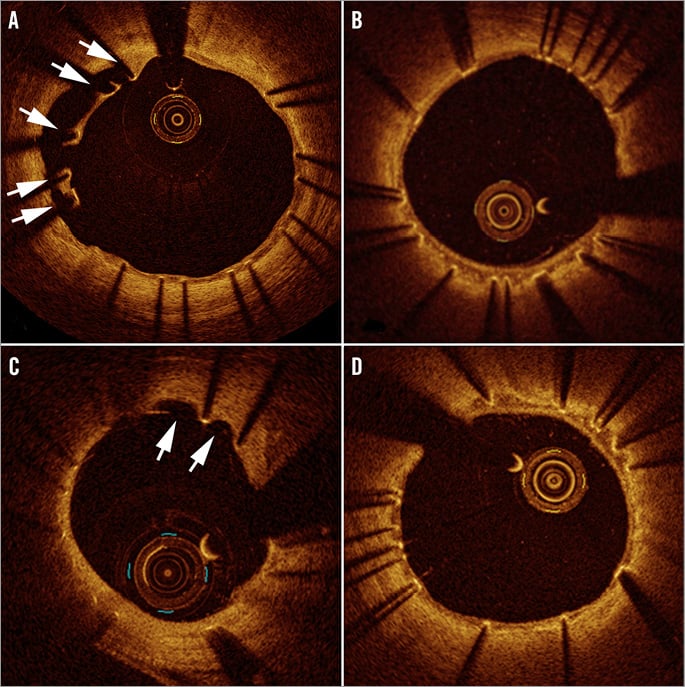
Figure 5. Apposition on optical coherence tomography. Incomplete stent apposition (white arrows) with the balloon-expandable stent (A) compared with absence of malapposition with the self-expanding STENTYS stent (B) at four-month follow-up. At nine months, evaginations with balloon-expandable stent (white arrows) (C) and embedded covered struts with self-apposing stent (D).
CLINICAL OUTCOMES
Clinical follow-up at one year was complete in 145 patients (95%). There were 12 MACE during follow-up: nine events occurred in the STENTYS group and three events in the control group (hazard ratio [HR] 2.13, 95% confidence interval [CI]: 0.58-7.85; p=0.37). The hazard ratios of other clinical events at one-year follow-up are shown in Appendix Table 6. Acute ST occurred in three STENTYS patients, possibly due to distal dissection and potential underexpansion during the index procedure, and probable late ST in one control patient. Cardiac death occurred in two patients in the STENTYS arm: one patient died due to index procedure-related complications resulting in cardiac tamponade and was not study device-related; one patient suffered sudden cardiac death at 48 days post procedure with unknown relation to target vessel or study device.
Discussion
In the present prospective, multicentre, single-blind, randomised, controlled trial, the self-apposing, sirolimus-eluting STENTYS stent compared with the balloon-expandable, zotarolimus-eluting stent in patients with STEMI undergoing PPCI resulted in significantly less malapposition and uncovered struts at four months after implantation and similar rates of apposition and coverage between groups at nine months. Luminal dimensions were significantly larger in the STENTYS group, with late loss being equivalent between groups, both at four and at nine months.
In the APPOSITION II trial, the self-apposing STENTYS BMS was tested against balloon-expandable stents and proved to be superior with respect to acute stent apposition, as assessed by OCT4. At three days post implantation, it was shown that the STENTYS mean stent area further increased, while the rate of malapposed struts decreased, suggesting that the stent conforms to changes in vessel anatomy during the first days after the index event. On a per-patient basis, none of the STENTYS stents was malapposed (defined as ≥5% malapposed struts) compared with 28% in the balloon-expandable stent group at three-day follow-up (p<0.001)4. The present trial extends these observations by assessing the midterm and long-term performance of the STENTYS SES and demonstrating that the acute and early stent apposition is maintained up to four and nine months after implantation, a finding potentially contributing to fewer adverse clinical outcomes7. Moreover, this trial shows that the lower rates of malapposition of the STENTYS stent translated into improved strut coverage at four months (both as strut coverage >20 µm as well as total stent coverage), compared with balloon-expandable stents, indicating early and faster healing of the vessel wall. A likely explanation for this observation is the mechanical property of the chronic outward force of the stent apposing the struts against the original intima of the vessel wall and thereby facilitating strut coverage by neointima.
The recommended duration of dual antiplatelet therapy (DAPT) after PPCI is six to 12 months8. However, interruption of DAPT is sometimes mandatory (surgery, gastrointestinal intervention) and exposes patients treated with a drug-eluting stent to a greater risk of ST. The higher rates of strut coverage of the STENTYS SES compared to control suggest a faster healing process that could reduce the risks associated with the temporary cessation of DAPT. Nevertheless, this hypothesis remains to be investigated in a randomised fashion.
Interestingly, in the nine-month cohort, no differences were observed between groups in terms of apposition and coverage, suggesting that the zotarolimus-eluting stent overhauled the gap with the STENTYS stent which was evident at four months, probably between six and nine months. The fact that 97% of all struts were covered (using a 20 µm cut-off) without malapposition with both the sirolimus-eluting STENTYS stent as well as the zotarolimus-eluting stent at nine-month follow-up may be considered a reassuring observation considering that ISA has been associated with ST due to delayed strut coverage of incompletely apposed stent struts9,10. Although numerous studies have demonstrated ISA of balloon-expandable stents in STEMI4,11-14, the present study did not confirm that.
In the present trial, the median LLL in the STENTYS group was 0.0 mm, indicating the increased effectiveness that results from the combined action of sirolimus and the self-expanding feature of the stent. The absence of any reduction in minimum lumen diameter combined with a very high rate of strut coverage is a good indicator of long-term safety for the STENTYS SES, considering that numerous studies have demonstrated LLL to be a predictor of future cardiovascular events. Another interesting finding is the augmented lumen size in the STENTYS arm. Previously, the APPOSITION II study showed that STENTYS implantation resulted in a larger lumen at three days: with the addition of sirolimus elution, this benefit is extended to four and nine months. Although the lumen areas were greater than the lumen reference areas on OCT in the STENTYS groups, the mean lumen (p=0.74) and mean stent areas (p=0.70) were comparable between the four- and nine-month STENTYS groups (data not shown), which demonstrates that the initial expansion of the stent levels off after a few months at a level somewhat larger than the reference area just outside the stent. Nevertheless, longer-term follow-up is needed to characterise the late vascular responses of the STENTYS self-expanding stent. Of note, post-procedural in-stent diameter stenosis with STENTYS was somewhat higher than controls. A potential explanation could be the larger reference diameter in the STENTYS group, possibly resulting from maximal expansion of the STENTYS stent.
Most recently, deferred stent implantation (median 9 hrs) after initial coronary reperfusion reduced no-reflow and increased myocardial salvage in high-risk STEMI patients compared to conventional PPCI with immediate stenting15. The rationale for delayed intervention in this study was to avoid the potential adverse effects of immediate stenting when the likelihood of no-reflow might be greatest. In this regard, the self-apposing feature of the STENTYS stent allows deployment with less barotrauma, potentially resulting not only in less intimal proliferation, but also less plaque disruption and/or thrombus dislodgement, thereby not negating immediate stenting during the index procedure. This feature may eventually lead to less distal embolisation, and hence could result in microcirculatory protection. However, in this study, no difference in TIMI flow or myocardial blush could support this hypothesis. The high frequency of post-dilation with large diameters at high pressures might have counterbalanced the potential beneficial effects of the STENTYS stent in this regard. Although it is possible that these measures are less sensitive than, for instance, the index of microcirculatory resistance, a larger trial is warranted to demonstrate definitely whether the STENTYS stent improves early microcirculatory function compared with conventional balloon-expandable stents.
Finally, although statistically not significant, numerically higher rates of adverse outcomes were observed in the STENTYS group. In this regard, despite procedure success rates being similar between groups, additional delivery system and device iterations were recently implemented to improve acute performance further. The learning curve for the operator may also be expected to play a role. Importantly, the present trial was not powered to discern differences in hard clinical endpoints. Whether the use of the self-expanding STENTYS stent can improve clinical outcomes in STEMI patients undergoing PPCI compared to balloon-expandable stents is currently being investigated in the ongoing international, multicentre, randomised APPOSITION V trial (ClinicalTrials.gov, Identifier: NCT01732341).
Conclusion
Among patients with STEMI undergoing PPCI enrolled in the present multicentre, randomised, controlled trial, the use of the self-expanding, sirolimus-eluting STENTYS stent resulted in lower rates of malapposition and uncovered struts at four months compared with the balloon-expandable, zotarolimus-eluting stent, as measured by OCT; however, it resulted in equivalent rates at nine months.
| Impact on daily practice The STENTYS stent exhibits better stent apposition compared with the balloon-expandable stent at four months with a greater percentage being fully covered, indicating early and faster healing of the vessel wall, and equivalent strut apposition and coverage at nine months. Furthermore, late lumen loss is equivalent between groups at four and nine months, with STENTYS SES showing no reduction in lumen diameter at nine months with a near perfect arterial healing. These results indicate that the addition of sirolimus elution to the STENTYS platform ensures fast healing and, potentially, an open vessel at longer-term follow-up after STEMI. |
Acknowledgements
The authors would like to thank Jürgen M. R. Ligthart for preparation of Figure5 and Katherin Awad for editorial assistance.
Funding
The APPOSITION IV study and associated data analysis were funded by STENTYS S.A., Paris, France.
Conflict of interest statement
R. Spaargaren is an employee of STENTYS. R.J.M. van Geuns has received institutional grants from Abbott Vascular, Boston Scientific, Tryton Medical and instructional speaker’s fees from Abbott Vascular, Boston Scientific, Tryton Medical and STENTYS SA. G. Montalescot reports receiving grants from Fondation de France, Sanofi-Aventis, Cordis, Medtronic, Boston Scientific, Fondation SGAM, during the conduct of the study; personal fees from Bayer, Boehringer-Ingelheim, CFR, Europa, GLG, Iroko Cardio International, Lead-Up, LLC, Luminex, McKinsey, Remedica, Servier, TIMI Group, WebMD, Wolters, and grants from Bristol-Myers Squibb, AstraZeneca, Biotronik, Eli Lilly, The Medicines Company, Medtronic, Menarini, Sanofi-Aventis, Pfizer, Accumetrics, Abbott Vascular, Daiichi-Sankyo, Nanospheres, STENTYS, outside the submitted work. W. Wijns has received institutional research grants from several device companies including Medtronic, STENTYS and St. Jude, and is a non-executive board member of Genae Ass. The other authors have no conflicts of interest to declare.
Appendix 1. Study organisation
Coordinating investigators: R.J.M. van Geuns, W. Wijns.
Clinical Events Committee: P.F. Agostoni, UMC Utrecht, Utrecht, The Netherlands; G.J. Laarman, Tweesteden Ziekenhuis, Tilburg, The Netherlands.
Data Safety and Monitoring Board: B. Rensing, Y. Appelman, R. Hoffmann, J.P. Tijssen.
Angiography/OCT Core lab: Cardialysis, Rotterdam, The Netherlands.
Site Management and Data Monitoring: Genae Associates, Antwerp, Belgium.
Appendix 2. Quantitative coronary angiography and optical coherence tomography
Quantitative coronary angiography (QCA) analysis was performed using the Coronary Angiography Analysis System (Pie Medical BV, Maastricht, The Netherlands). In each patient, the stented segments, along with 5 mm proximal and distal to the stent edges, were analysed. The QCA measurements included RVD, calculated with an interpolation method, minimal lumen diameter (MLD), and percentage diameter stenosis. Binary restenosis was defined in every segment as diameter stenosis ≥50% at follow-up. Late lumen loss (LLL) was defined as the difference between post-procedure MLD and MLD at follow-up.
The detailed OCT methodology has been previously reported4. In brief, intracoronary OCT imaging using the C7XR FD-OCT Imaging System (LightLab Imaging, Westford, MA, USA) with an RX ImageWire II catheter (LightLab Imaging) was performed at the end of the procedure with a pullback speed of 20 mm/s and an image acquisition at 100 frames/s. A dedicated semi-automated contour-detection system (QCU-CMS; Medis medical imaging systems bv, Leiden, The Netherlands) was used to delineate the lumen contours of each cross-sectional image. Cross-sections, in which lumen, stent, and incomplete stent apposition (ISA) areas were calculated, were analysed at each 1 mm interval along the entire stented segment and 5 mm proximal and distal. Standard definitions of cross-sectional area and volume measurements, including strut apposition and tissue coverage16, were applied as previously reported4,17. Specifically, malapposition was defined as a distance between strut endoluminal surface and the vessel wall greater than the strut thickness and was considered present if at least one single strut was incompletely apposed to the vessel wall. The struts were classified as uncovered when there was no visible tissue covering its surface. Tissue coverage was measured per strut as the distance from strut mid-endoluminal surface to lumen2 and expressed as % covered struts using a cut-off of both 0 µm and 20 µm.
Appendix 3. Enrolment
The Netherlands: (total enrolment=58): Thoraxcenter, Erasmus Medical Center, Rotterdam - R.J.M. van Geuns (26); AMC, Amsterdam - K.T. Koch (15); Albert Schweitzer Hospital, Dordrecht - A. IJsselmuiden (9); OLVG, Amsterdam - G. Amoroso (8).
France: (total enrolment=34): CHU Gabriel Montpied, Clermont-Ferrand - G. Souteyrand (19); Clinique Saint-Hilaire, Rouen - J. Berland (7); CHU La Pitié-Salpêtrière, Paris - G. Montalescot (5); CHU Henri Mondor, Créteil - E. Teiger (3).
Italy: (total enrolment=34): Ferrarotto Hospital, Catania - C. Tamburino (34).
Belgium: (total enrolment=24): Ziekenhuis Oost-Limburg, Genk - M. Vrolix (14); OLV Aalst, Aalst - W. Wijns (10).
Denmark: (total enrolment=2): Aarhus University Hospital, Skejby - H. Christiansen (2).