- STEMI
- IVUS
- bare metal stent
- OCT
Abstract
Aims: In the setting of ST-elevation myocardial infarction (STEMI), epicardial vasoconstriction and thrombus load may lead to stent undersizing and malapposition after primary percutaneous coronary intervention (PPCI), which can both be responsible for stent thrombosis or restenosis. Aggressive stent deployment can, on the other hand, cause distal embolisation and the no-reflow phenomenon. The purpose of our study was to evaluate the safety and feasibility of a novel self-expanding stent by assessing the clinical, angiographic and intravascular outcomes after stent deployment at three days and at six months follow-up.
Methods and results: This prospective, multicentre, non-randomised study enrolled 25 STEMI patients undergoing PPCI; a nitinol, self-expanding, coronary stent (STENTYS® stent; STENTYS, Paris, France) was used in all patients. Angiography and intravascular ultrasound (IVUS) or optical coherence tomography (OCT) were performed immediately after stent deployment, after three days and at six months. Primary safety endpoints were mortality, reinfarction, stent thrombosis and stroke at discharge and at six months. The primary feasibility endpoints were technical, device and procedural success, and stent apposition at three days and six months. Secondary endpoints included distal embolisation, binary restenosis, ischaemia-driven target lesion revascularisation (TLR) and late lumen loss (LLL). There were no adverse events at discharge or at six months. Technical, device and procedural success were 100%, 96% and 96%, respectively. IVUS showed a significant vasodilatation distal to the culprit lesion at three-day follow-up (+19%), with a concordant expansion of the implanted stent (+18%), p≤0.001 for both values. One case of distal embolisation was reported. There were no cases of late stent malapposition at six months. In-stent and in-segment LLL were 0.71±0.71 mm and 0.58±0.61 mm. Binary restenosis was 25%, ischaemia-driven TLR was 12%.
Conclusions: This study shows that the use of the STENTYS® self-expanding stent is safe and feasible in STEMI patients. Three days after the procedure, the stent expanded to the same extent as the epicardial vasodilatation and appeared completely apposed to the vessel wall. This could be of benefit in preventing stent thrombosis in the setting of STEMI.
Abbreviations
BMS bare metal stent
DES drug-eluting stent
DS diameter stenosis
ISA incomplete stent apposition
IVUS intravascular ultrasound
LLL late lumen loss
MLD minimal lumen diameter
OCT optical coherence tomography
PPCI primary percutaneous coronary intervention
QCA quantitative coronary angiography
RVD reference vessel diameter
STEMI ST-elevation myocardial infarction
TIMI Thrombolysis In Myocardial Infarction
TVR target vessel revascularisation
Introduction
Primary percutaneous coronary intervention (PPCI) is considered the optimal approach for the treatment of ST-elevation myocardial infarction (STEMI), because of its superior reperfusion outcomes when compared to thrombolysis1-3.
The presence of thrombus and epicardial vasoconstriction, which is only partially responsive to nitrates, can lead to an underestimation of the vessel size and consequently to implantation of an undersized stent and late stent malapposition. In the acute setting, stent undersizing is one of the most important predictors of stent thrombosis4,5. Studies have shown that both BMS and DES can safely be used in PPCI for STEMI6-11. However, while the short-term malapposition rates of BMS and DES appear to be similar (32.7% and 35.2%, respectively, in the HORIZONS-AMI IVUS substudy12), one study showed a higher rate of late stent malapposition after DES implantation, raising concerns about the long-term safety of DES use in patients with STEMI13.
However, aggressive stent deployment and oversizing can lead to plaque/thrombus disruption and distal embolisation. The subsequent occurrence of poor myocardial reperfusion (no-reflow phenomenon) leads to a lack of myocardial salvage with poor short- and long-term clinical outcomes14.
In vessels with a high thrombus load and major vasoconstriction, such as in STEMI, the feature of self-expansion could potentially reduce the occurrence of stent malapposition and distal embolisation, and thus of stent thrombosis and no-reflow.
The purpose of our study was to evaluate the safety and efficacy of the STENTYS® self-expanding stent in STEMI patients by assessing the clinical, angiographic and intravascular outcomes acutely at three days and at six months post-procedure.
Methods
The STENTYS® stent
The STENTYS® coronary stent is a self-expanding, nitinol, bare metal stent with a nominal strut width of 0.0027’’ (68 microns) (Figure 1). The available stent lengths in the study were 22 and 27mm with a diameter suitable for vessels with a reference vessel diameter (RVD) between 3.0 and 3.5mm. A 6Fr compatible, rapid-exchange delivery system delivers the stent into position over a conventional 0.014” guidewire, and the stent is deployed by the withdrawal of aretractable sheath. The stent has a Z-shaped design that is linked together by small interconnections, which can be disconnected by balloon inflation between the struts to create side branch access, if needed.

Figure 1. STENTYS® self-expanding stent.
Study design
We conducted a multicentre, prospective, non-randomised, single-arm study. The primary objective was to assess the safety and feasibility of the STENTYS® stent in STEMI patients.
The study protocol was approved by local ethics committees and competent authorities, and was conducted in accordance with the Declaration of Helsinki. All serious adverse events were adjudicated by an independent clinical events committee. All angiographic, IVUS and OCT films were analysed by an independent core lab (Cardialysis, Rotterdam, The Netherlands). All data was monitored by a monitoring organisation (Medpass, Paris, France) and entered into a database (double data entry). All patients provided written informed consent.
Patient population
Patients admitted for STEMI with an onset of chest pain of less than 12 hours were screened for enrolment in the study. The inclusion criteria were: >20 minutes of chest pain and ≥1mm of ST-segment elevation in at least two contiguous leads or new left bundle branch block; age between 18 and 80 years; a de novo native coronary lesion suitable for PCI with primary stent implantation; RVD ≥3.0mm and ≤3.5mm; target lesion length ≤ 21mm (visually estimated) in order to be covered by a single study stent.
The exclusion criteria were: prior thrombolytic therapy; myocardial infarction caused by in-stent restenosis or restenosis; cardiogenic shock; contra-indication to aspirin or clopidogrel; uncertain neurological outcome after cardiopulmonary resuscitation; patients under mechanical ventilation; renal failure (creatinine >2.5mg/dl or 150µmol/l); known hypersensitivity to nickel-titanium; uncorrected bleeding disorders; previous stenting of the target vessel; unprotected left main coronary disease with >50% stenosis; trifurcation lesions; excessive tortuosity of the target vessel; coronary artery bypass graft surgery (CABG) planned for the target or another vessel; participation in another investigational drug or device study where the follow-up period was incomplete.
Study procedure and medication
Patients were treated according to the current daily practice in each of the participating centres. Thrombo-aspiration was recommended, while predilatation was left to the discretion of the investigator. Intracoronary nitroglycerine was systematically administered after thrombus aspiration in order to properly evaluate vessel size. Stent disconnection to allow side branch access was recommended if the diameter of the side branch was >2.25mm with TIMI flow <3, and/or stenosis >50%, and/or dissection >grade B. The use of post-dilatation was recommended if there was a residual stenosis >30%.
Before the procedure, patients received anticoagulation therapy according to local practice. The protocol recommended a 500mg bolus of aspirin, 300-900 mg clopidogrel, a heparin bolus of 5,000IU IV and the use of glycoprotein IIb/IIIa inhibitors. Use of bivalirudin was accepted. After the procedure, patients received aspirin (at least 75mg/day indefinitely) and clopidogrel (75mg/day for at least one month after the procedure).
Follow-up timelines
Clinical data was collected before and after the procedure, at discharge and at six months. Angiographic and IVUS assessments were performed immediately after stent implantation, at three days after the procedure, and at six months post-procedure. In a subgroup of patients, OCT was performed instead of IVUS for optimal assessment of the presence of thrombus and for stent apposition.
Study endpoints
Primary safety endpoints were all-cause mortality, reinfarction, stent thrombosis and stroke at discharge and at six months. Primary efficacy endpoints were technical, device and procedural success after stent placement, and apposition of the stent at three days and six months. Secondary efficacy endpoints were angiographic signs of distal embolisation after stent placement (investigator assessment), binary angiographic restenosis, late lumen loss (LLL), and ischaemia-driven target lesion revascularisation (TLR) at six months.
Definitions
All deaths were considered cardiac unless a non-disputed non-cardiac cause was present. A TLR was considered clinically indicated if angiography at follow-up showed both a diameter stenosis ≥50% (core lab QCA assessment) and one of the following: (1) a positive history of recurrent angina pectoris, presumably related to the target vessel; (2) objective signs of ischaemia at rest (ECG changes) or during exercise test (or equivalent), presumably related to the target vessel; (3) abnormal results of any invasive functional diagnostic test (e.g., Doppler flow velocity reserve, fractional flow reserve); (4) a TLR or target vessel revascularisation (TVR) with a diameter stenosis ≥70% even in the absence of the above-mentioned ischaemic signs or symptoms. Technical success was defined as the ability to cross with the device and deploy the stent as intended at the target lesion. Device success was defined as technical success with achievement of a final diameter stenosis <30%, and with TIMI flow 2 or 3 by quantitative coronary angiography (QCA). Procedural success was defined as technical success without the occurrence of death, reinfarction or repeat revascularisation of the target lesion during the hospital stay.
Angiographic assessment
Quantitative coronary angiography (QCA) was performed using the CAAS5 analysis system (Pie Medical BV, Maastricht, The Netherlands)15. In each patient, the stented segment and the peri-stent segments (defined by a length of 5mm proximal and distal to the stent edge) were analysed. Repeat angiography was scheduled at three-days and six-month follow-up.
Quantitative analysis of all angiographic data was performed offline by an independent core laboratory (Cardialysis, Rotterdam, The Netherlands). The following QCA parameters were determined: computer-defined minimal luminal diameter (MLD), reference diameter obtained by an interpolated method, and percentage diameter stenosis. Binary restenosis was defined in every segment as diameter stenosis ≥50% at follow-up. Late loss was defined as the difference between MLD post-procedure and MLD at follow-up16.
IVUS assessment
Intravascular ultrasound images were acquired by motorised pullback at a constant speed of 0.5mm/s. A computer-based contour detection program (Curad BV, Wijk bij Duurstede, The Netherlands) was used for automated three-dimensional reconstruction of the coronary segment beginning 5mm distal and extending 5mm proximal to the stented segment. Feasibility, reproducibility and inter- and intra-observer variability of this system have been validated in vivo17,18.
Quantitative analysis of all IVUS data was performed offline by an independent core laboratory (Cardialysis BV, Rotterdam, The Netherlands). The lumen, stent boundaries and external elastic membrane (vessel boundaries) were detected using a minimum cost algorithm. The stent volume (SV) and lumen volume (LV) were calculated by multiplying length by mean areas. The intra-stent neointimal volume was calculated as the difference between SV and LV. The percentage obstruction of the stent volume was calculated as the intra-stent neointimal volume/stent volume*100.
Incomplete stent apposition (ISA) was defined as one or more stent struts separated from the vessel wall with evidence of blood speckles behind the strut on ultrasound. Late incomplete stent apposition was defined as ISA at follow-up that was not present post-procedure19.
OCT assessment
Optical coherence tomography acquisitionOCT acquisition was carried out in five patients with the C7-XR™ Fourier-domain system (LightLab Imaging, Westford, MA, USA) using the flushing technique.
These acquisitions were performed by first advancing a conventional wire distal to the segment of interest, and then advancing the OCT imaging catheter (RX ImageWire II; LightLab Imaging) distally towards the treated region. Pullback was performed while contrast medium (3mL/s, Iodixanol 370, Visipaque; GE Healthcare, Cork, Republic of Ireland) was injected continuously into the guide catheter using an injection pump. In this case, the automated pullback rate was 20mm/s and the frame rate was 100 images/s.
Optical coherence tomography analysisOCT measurements were performed by an independent core lab (Cardialysis, Rotterdam, The Netherlands) with proprietary software for offline analysis (LightLab Imaging). The analysed region comprised the stented segment as well as the segments 5mm proximal and distal to the stent. The lumen and stent areas were measured at 1mm intervals.
In order to determine apposition of the struts, the following was performed: (1) the distance from the middle point of the endoluminal side of each strut to the lumen contour was measured; (2) if this distance was greater than the strut thickness, this was considered a malapposed strut; (3) in the frames with malapposed struts, the difference between the lumen and stent areas was measured, as this represented the incomplete stent apposition area.
Statistical analysis
Analyses were conducted on an intention-to-treat basis. Continuous variables were summarised by mean and standard deviation. Descriptive statistics were provided for all variables considered in the analysis. Categorical variables are presented as counts and percentages. Continuous variables are presented as mean, standard deviation, and number of observations. Statistical analysis was performed using Statistical Analysis System (SAS) for Windows (version 9.1; SAS Institute Inc., Cary, NC, USA). Paired analysis (comparisons of results for individual patients immediately post-stent placement and at follow-up) was performed for IVUS data.
Results
A total of 25 patients (mean age 58 years; 60% males) were enrolled between March and October 2009 at five European sites. In 20/25 patients, IVUS was performed following stent placement, while in the remaining five patients, OCT was performed.
Procedural and in-hospital results
Table1 summarises the subject and lesion characteristics at baseline. TIMI flow 0 at baseline was present in 68% of all subjects.
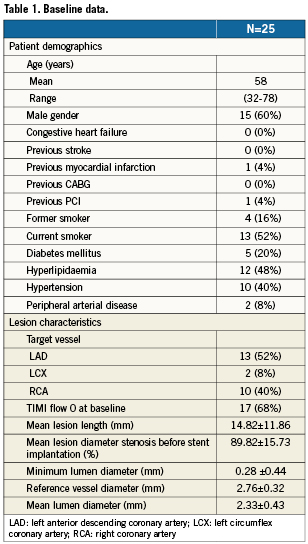
Thrombo-aspiration before stent implantation was performed in 17/25 patients (68%), and 14/25 patients (56%) required predilatation of the culprit lesion. Ten patients received GPIIb/IIIa inhibitors.
Technical success was 100% (25/25 patients). In five patients, an additional, non-study stent was used (one BMS and four DES); in four cases, this second stent was placed to treat a second lesion, and in one case because of insufficient lesion coverage. Final TIMI3 flow after stent implantation was achieved in 24/25 patients (96%); there was one angiographic finding of distal embolisation and TIMI2 flow. This patient presented with a 99% stenosis in the LAD with TIMI0 flow; thrombectomy, pre- and post-dilatation were performed and IIB/IIIA inhibitors were administered.
In 20/25 patients (80%), postdilatation was performed: in 11 patients at pressures ≤15 ATM; in nine patients at high pressures (above 15 ATM).
Device success was 96% (24/25 patients) as one patient had a residual stenosis of 52% due to a heavily calcified lesion. Procedural success was 96% (24/25 patients) with no stent thromboses or adverse events prior to discharge.
Six-month clinical and angiographic results
Clinical follow-up at six months was available in 25/25 patients. No death (cardiac or non-cardiac), reinfarction or stent thrombosis was reported at six months post-procedure. One patient experienced a reversible ischaemic neurological deficit 140 days post-procedure.
Angiographic follow-up was available for 20/25 patients. Five patients, all free of symptoms, refused to undergo the scheduled catheterisation.
There were no late stent thromboses or total occlusions; 5/20 patients (25%) showed angiographic binary restenosis. Three of them had clinically-driven TLR (12%), while two patients had non-clinically driven TLR (restenosis <70% with no symptoms of ischaemia).
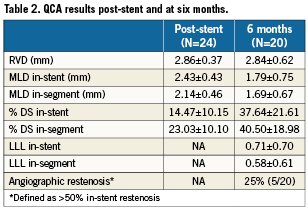
In-stent LLL was 0.71±0.71 mm and in-segment LLL 0.58±0.61mm. No stent fractures were detected. QCA results are shown in Table2.
IVUS assessment
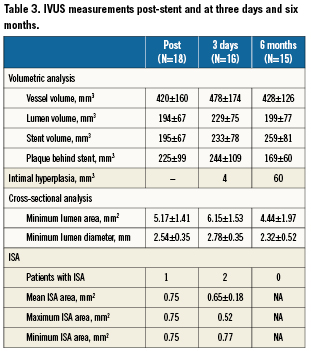
IVUS was performed after stent implantation, at three days, and at six months after the procedure in 20/25, 16/25 and 15/25 patients, respectively. Table3 shows the results of those IVUS controls.
Three-day, paired IVUS results showed a non-significant increase of the proximal segment (+5%), but a significant increase of the mean reference area distal to the stent (+19%; p<0.001). Mean stent area and minimum lumen area also significantly increased (+18% and +19%, respectively; p<0.001 for both). Table4 provides the mean changes in MLA of the patients who had IVUS follow-up; Figure2 gives an overview of the MLA changes of individual patients.
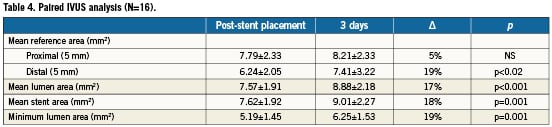
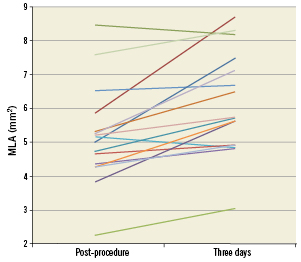
Figure 2. Paired IVUS analysis: MLA post-procedure compared to MLA at three days for individual patients.
Incomplete stent apposition (ISA) was present in one patient (5%) post-stent placement. None of the patients had ISA at six-month follow-up. An example of stent expansion is illustrated in Figure3.
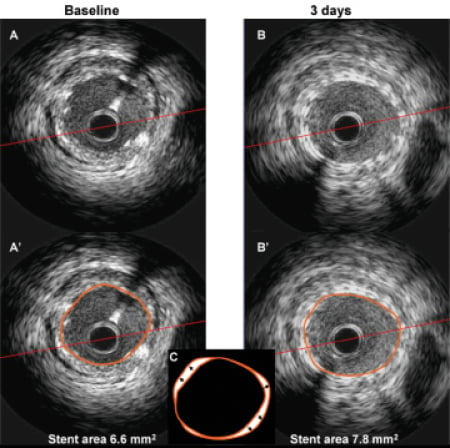
Figure 3. IVUS images at baseline (A and A’) and at three-day follow-up (B and B’), showing an 18% increase in stent area. In panel C, the two stent contours are overlapped. Arrows indicate the increase in stent size over three days.
OCT assessment
OCT was performed after stent implantation, at three days, and at six months after the procedure in 5/25, 4/25 and 3/25 patients, respectively.
Paired OCT results showed a significant increase of minimal lumen area at three days (5.52±1.46mm2 to 7.50±0.73mm2; +28%). The percentage of malapposed stent struts immediately after the procedure was 2.27% and decreased to 0.11% three days later. Figure4 demonstrates that some malapposition immediately after stent implantation has resolved at three-day follow-up. This full apposition is the consequence of an increase of the stent area following resolution of vessel constriction in the setting of acute MI.
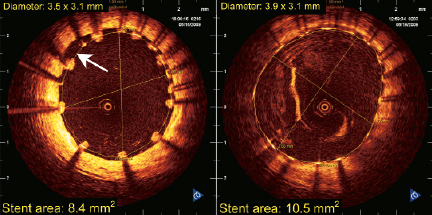
Figure 4. OCT images immediately after stent implant (left) and after three-day follow-up (right). Malapposition area between 8 and 10 o’clock (white arrow) present after stent implant has resolved three days later.
Discussion
This study shows that the use of the self-expanding STENTYS® stent is safe and feasible in the setting of PPCI for STEMI. This stent also displays the distinctive feature of positively adapting to the changes in coronary anatomy (vasodilatation, thrombus dissolution), which occur during the first few days after the procedure.
Stent thrombosis is one of the major unresolved issues in PPCI. In this setting, stent thrombosis rates are two to three times higher than in the case of elective procedures. The HORIZONS-AMI study13 demonstrated a 3.3% stent thrombosis rate (definite/probable) at one year. Its occurrence was associated with various factors, both patient and procedure-related.
Epicardial coronary vasoconstriction due to systemic adrenergic activation during PPCI can lead to an underestimation of the reference vessel diameter with subsequent implantation of an undersized stent. Furthermore, residual intracoronary thrombus after thromboaspiration may later dissolve, leaving a gap between the stent and the vessel wall. Stent undersizing and malapposition are both potential causes of stent thrombosis5,6.
To our knowledge, this is the first study in the setting of STEMI with IVUS examinations performed both immediately after stent implantation and at three days. We demonstrated a significant vasodilatation distal to the culprit lesion at three days after the procedure (+19%). Our results confirm the occurrence of severe epicardial vasoconstriction in the acute setting of STEMI and underscore the need for developing techniques or devices to avoid stent undersizing. The concordant increase in the STENTYS® stent area (+19%) at three days and the absence of malapposed stents seen at six months suggest that this device follows the growth of the vessel lumen while vasoconstriction and thrombus are resolving.
Studies have suggested that neointimal proliferation after stent implantation is proportional to vessel wall injury caused by the force exerted on the arterial segment20. Vessel wall injury with self-expanding stents is different from that of balloon-expandable stents. A nitinol self-expanding stent tends to take its original memory shape. The radial force of self-expanding stents decreases when the stent diameter increases, reaching an equilibrium when the outward force of the stent equals the inward force of the vessel. The STENTYS® stent has a memory diameter more than one millimetre beyond the size of the vessel that it is intended for; this means that the self-expanding stent automatically adapts to the size of the vessel.
At six-month follow-up, this is translated into regression of the plaque behind the struts together with an increased growth of the neo-intima. Therefore, with self-expanding stents, late stent expansion and vessel growth is offset by larger intimal proliferation. The net result is that balloon-expandable and self-expanding stents result in similar values for mean and minimal lumen diameters, and have equivalent LLL at long-term follow-up.
The ability of a self-expanding stent to grow in volume in the first hours to days after the procedure allows gentle deployment with less trauma, but also reduces plaque disruption/thrombus dislodgement and thus could lead to less distal embolisation21,22. In our study we observed one case of angiographically visible distal embolisation, and a very high percentage of patients achieved final TIMI3 flow (96%). Animal studies have also confirmed that this stent does not need to be deployed at high pressures as it continues to expand after implantation23.
The STENTYS® stent differs from other self-expanding and nitinol stents in several ways. While the WALLSTENT® (Boston Scientific, Natick, MA, USA) was a self-expanding stent, it was not made of nitinol but a braided, cobalt-alloy wire. It had clinical and mechanical shortcomings including difficulties in precise positioning as a result of foreshortening of up to 20% on expansion, and sharp wire ends that could lead to vessel trauma24.
Unlike the WALLSTENT® but similar to the STENTYS® stent, the Radius™ stent (Boston Scientific) was a nitinol stent, laser-cut from a tube. However, the Radius stent had a completely different design compared with the STENTYS® stent, resulting in different mechanical properties such as different radial and chronic outward forces, thicker and longer struts, and a larger cell size that is not optimised for conformability to vessel wall variations21,22. As aresult of these technical shortcomings and poor outcome, clinical use of previous self-expanding stents has been limited.
The STENTYS® stent showed favourable behaviour in terms of LLL. The binary restenosis rate was 25%, compared to the 22.9% restenosis at 13-month follow-up reported in the BMS arm of the HORIZONS trial in similar lesions of similar lengths and comparable RVDs13. The clinically-driven TLR rate was low, thus supporting the hypothesis that the use of drug-eluting stents, taking into account their potential long-term caveats, is not mandatory in the case of PPCI.
Bifurcation lesions remain a challenge and have been reported in over 20% of patients treated with PPCI25. Outcome data for PPCI of bifurcation lesions is limited, since STEMI is a common exclusion criterion in randomised bifurcation studies26. In one observational study, the presence of bifurcation lesions in the setting of PPCI caused a slight excess of MACE23, while in the NORDIC study, PPCI with DES stenting of bifurcation lesions resulted in a trend towards an excess of stent thrombosis27. The larger CADILLAC trial confirmed a higher incidence of stent thrombosis in bifurcation lesions following PPCI (29% vs. 5% in non-bifurcations; p=0.002)28. In the APPOSITIONI study, only one patient required treatment of a bifurcation. One of the features of the STENTYS® stent is the possibility of disconnecting struts to create access to a side branch. This was done with good results in one study patient. Although adedicated feasibility study has shown the potential benefits of the use of the STENTYS® stent in bifurcation lesions29, it remains to be demonstrated whether this also applies to the treatment of bifurcation lesions in the setting of PPCI.
Limitations
This was a non-randomised, controlled feasibility study with a small number of patients and strict selection criteria. Thus, these results cannot be extrapolated to broader patient groups with wider selection criteria. As it was a feasibility study, the trial was not intended to enable firm conclusions on the safety and efficacy of the device to be drawn.
Conclusions
This APPOSITION I feasibility study shows that the use of the STENTYS® self-expanding stent is safe and feasible in the treatment of STEMI patients. Three days after the procedure, the stent expands further to match the vasodilatation occurring distal to the culprit lesion, and shows excellent apposition at six months. Whether these findings could result in a lower late stent thrombosis remains to be determined by further studies.
Acknowledgements
The authors would like to acknowledge all the cardiologists involved in the PCI procedures of APPOSITION I. The authors would also like to acknowledge the contribution of the Clinical Event Committee consisting of P. Agostoni, Department of Cardiology, University Medical Centre, Utrecht, The Netherlands and E. Kedhi, Department of Cardiology, Maasstad Ziekenhuis, Rotterdam, The Netherlands.
Conflict of interest statement
R. Spaargaren is an employee of Stentys. The other authors have no conflicts of interest to declare.