
As in other medical fields, there are some recurring topics in the coronary stent area that emerge after every new finding or innovation. Incomplete stent apposition (ISA) is one of those recurring topics. However, the very concept of ISA brings together very different situations. In recent years, late acquired ISA has been a subject of research, strongly associated with late and very late drug-eluting stent thrombosis, the underlying cause being arterial wall inflammation and consequent remodelling1-3. On the other hand, there is also acute ISA. Several studies have concluded that it is not so uncommon and that it can either remain or regress over time. Regarding its clinical implications, follow-up studies in patients with acute ISA have failed to identify ISA as a predictor of either bare metal stent or drug-eluting stent thrombosis4,5. Nevertheless, a necropsy study reported a high acute ISA incidence in thrombosis cases of stents implanted in acute coronary syndromes (34% in thrombosed stents vs. 18% in patent stents, p=0.008)6. Potential linking factors could be induced shear stress and delayed tissue coverage7. Clearly, the differences observed between these studies can be easily explained by the different methodology. In any case, acute ISA is more frequent when stents are implanted in the ST-elevation myocardial infarction (STEMI) setting due mainly to an undersizing of the stent (thrombus and vasoconstriction)8.
Technical innovations aimed at reducing ISA incidence are not a novelty. A self-expanding stent was studied in the 1990s, but it was abandoned due to the high restenosis rate. The idea was later revived, bearing in mind what had been learnt in those earlier days, and a neatly improved self-expanding stent model emerged, namely the STENTYS Self-Apposing® stent (STENTYS S.A., Paris, France). Taking into consideration the previously noted higher rate of acute ISA in the STEMI setting and given that its clinical impact might be enhanced in this population, the STENTYS stent has been mainly assessed in this context. A previous randomised trial (APPOSITION II) showed the superiority of this stent compared with balloon-expandable stents in post-procedural strut apposition9. Obviously, more ample studies are needed to evaluate whether superior strut apposition would indeed lead to improvement in clinical outcomes in a contemporary STEMI patient population.
The paper published in this issue of EuroIntervention by Nakatani et al addresses this topic in a very detailed and neat way10. This is a substudy from the previously mentioned APPOSITION II trial where investigators analyse in greater depth the incidence and potential mechanism of resolved, persistent and newly acquired malapposition revealed in the study. The current analysis includes 69 patients (self-expanding [SE]: 35, balloon-expandable [BE]: 34) with serial OCT studies post procedure and three days after.
Given the goal of the study, it was important to match the regions with ISA at different time points in order to assess the temporal changes. The ideal strut-by-strut matching at two time points is practically impossible to perform due to the rotation of the catheter and limited longitudinal resolution. Therefore, the investigators resorted to a segmental level matching using fiduciary landmarks. In addition, foldout views based on frame-by-frame analysis were created to depict the clustering and spread distribution of ISA.
The authors proposed the following as hypothetical mechanisms for the dynamic changes in ISA: stent recoil, stent enlargement, vasorelaxation, tissue resorption and tissue formation. The cut-off value of 0.7 mm2 for recoil or enlargement was derived from previous studies reporting the standard deviation of the absolute inter-observer difference in the stent area analysis and the 95% confidence interval of the absolute inter-pullback difference in the stent area analysis.
ISA which resolved at three days without stent enlargement was considered to be due to tissue formation behind the malapposed struts, and ISA volume which increased at three-day follow-up in the absence of an “early” recoil and/or vasorelaxation was judged to be caused by tissue resorption. The authors found a higher acute, persistent and newly acquired ISA incidence in the BE-stent cohort compared to the SE-stent cohort. In the SE group, 53.6% of acute ISA were resolved as opposed to 23.5% in the BE group. New appearances of ISA were caused by tissue resorption, vasorelaxation and “early” recoil in balloon-expandable stents and only tissue resorption in self-expanding stents. Figure 1 shows the different types of ISA, both post procedure and three days after. Mechanisms leading to each type of ISA in relation to the stent model are also described.
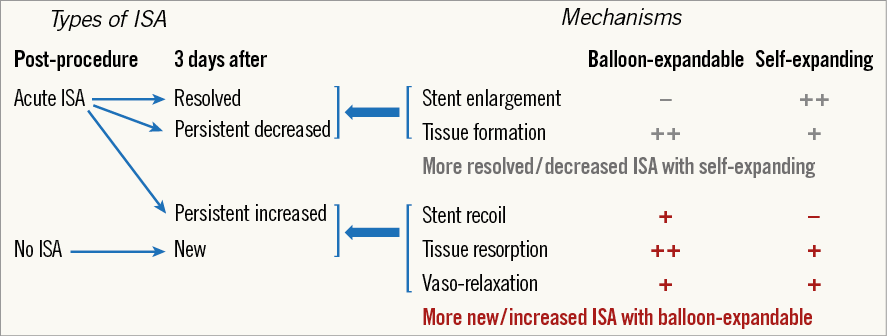
Figure 1. Types of ISA and mechanisms leading to each type of ISA in relation to the stent model. ISA: incomplete stent apposition
Several issues are worthy of consideration. Post-dilatation was significantly more frequent in the SE group, even though the mean stent area post-procedure was smaller. The need to post-dilate these devices has been clearly pointed out in the recently published APPOSITION III registry, in which cases with post-dilatation revealed a lower definite thrombosis rate (1.9% at one year) compared to cases without it (5% at one year) (p=0.01)11. On the other hand, with regard to differential ISA changes, the discrepancy in post-dilatation might have had an impact on the findings by means of inducing a greater thrombus crushing and embolisation, leading to a larger arterial wall disruption and a larger strut penetration in the necrotic core. All these factors might partially explain the different tissue activity observed between the two stent models.
Another aspect to consider is the degree of recoil observed. It is striking to find a non-negligible degree of recoil with BE stents in STEMI culprit plaques of 59-year-old (mean) patients with only 12% of diabetics and therefore with a low presence of fibro-calcified plaques. This makes us reflect on the technical information of stents obtained from ex vivo studies.
A further key point is the clinical impact of these findings. We have already indicated that the relationship between acute ISA and acute or subacute thrombosis is not sufficiently clear. In fact, there have to be other factors, as proven by the lower acute/subacute thrombosis rate of everolimus-eluting stents compared to the same metallic platform of bare metal stents in patients with infarction, when one would have expected the same degree of ISA for both.
Another adverse consequence of persistent ISA could be the delay in or prevention of endothelialisation of the struts. We already know it depends on the extent of ISA. A previous study has shown that an ISA detachment >300 μm is associated with 15.7% of struts still being uncovered at six-month follow-up7. In the study published in this issue, the maximum ISA detachment distances associated with persistent ISA were >250 μm for SE stents and >200 μm for BE stents. This indicates that ISA cases more prone to relate to delayed endothelialisation are at the same time more difficult to regress even with self-expanding stents. On the other hand, the degree of the maximum area of persistent ISA with balloon-expandable stents (0.6 mm2) is much lower than the areas of ISA found in cases with late drug-eluting stent thrombosis.
Having said this, this study analyses in a very deep, precise and correct manner the topic of ISA and its dynamic evolution in the short term with the balloon-expandable and the self-expanding platforms. The benefits of the latter are well established; it remains to be determined whether this can be translated into a positive clinical impact. Therefore, it seems very appropriate to perform more ample studies such as the APPOSITION V trial12.
This study is a good example of how to perform a proper assessment of a new stent design. We have already seen an even greater scrutiny with bioresorbable scaffolds, and we should hope to see the same with every new model. After all these mechanistic studies, the appropriate implementation of an ambitious enough clinical study programme should always follow.
Conflict of interest statement
The author has received unrestricted research grants from Abbott Vascular, Boston Scientific, Biotronik, Biosensors, St. Jude Medical.

