- OCT
- angioplasty
- drug eluting stent
- strut apposition
Abstract
Aims: To analyse the relationship between strut apposition as visualised with optical coherence tomography (OCT) at follow-up and clinical and procedural characteristics at stent implantation, and to examine the relationship between strut apposition and stent healing.
Methods and results: Twenty-eight coronary lesions were evaluated. The lesion morphology before stent implantation was described from the baseline angiogram. Strut apposition at follow-up was divided into: (I) apposed struts, (II) struts overlying side branch ostia, (III) malapposed and (IV) protruding struts. Since malapposed and protruding struts often occurred in the same lesions, these were divided into two groups: lesions without (n=20) and lesions with (n=8) the presence of these struts. The thickness of strut coverage was used as a surrogate for stent healing. We analysed 5,159 struts. Sixteen were malapposed and 216 were protruding. Lesions with malapposed and/or protruding struts at OCT follow-up were more frequently associated with acute coronary syndrome (ACS) and procedure related dissections at stent implantation than lesions without. There was a tendency towards a less pronounced strut coverage over malapposed and protruding struts, as compared to apposed struts.
Conclusions: ACS and procedural dissections at stent implantation may be related to strut malapposition/protrusion at follow-up, which may influence the degree of strut coverage.
Introduction
Late coronary stent malapposition has been reported after bare metal stent (BMS) as well as drug-eluting stent (DES) implantation, using intravascular ultrasound (IVUS).1,2 Although stent malapposition has been related to late stent thrombosis (LST)3,4, the results of other studies suggest malapposition to occur in up to 10% of implanted DES without any clinical consequence at late follow-up.5,6 The importance of late stent malapposition therefore remains controversial.
Optical coherence tomography (OCT) is an infrared light-based imaging technique that provides detailed information about the coronary vessel wall, strut apposition and tissue coverage in vivo.7 With a resolution of 10 μm, OCT detects strut malapposition and strut coverage more efficiently than IVUS.8
We have recently suggested the following OCT based classification of strut apposition: (I) apposed struts, (II) struts overlying the ostium of a side branch, (III) malapposed struts, and (IV) pseudoapposed/protruding struts (Figure 1).9 Protruding struts were found in frames with ”corrugated” lumen contours, and were characterised by finding that “pits” in the lumen contour extended beyond the stent area trace delineated from the reconstructed abluminal strut surfaces, although struts appeared to be well apposed to the vessel wall. Protruding struts were often present in frames and lesions containing malapposed struts, and were frequently surrounded by structures displaying a lower signal-intensity than the rest of the vessel wall. The nature of these structures is not known, but morphologically they resemble fibrin deposits that separate malapposed struts from the vessel wall, as seen in previously published histology images.9,10
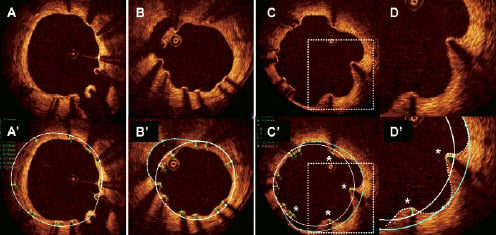
Figure 1. Classification of strut apposition. Apposition was evaluated after reconstruction of strut thicknesses (green traces) according to manufacturers’ specifications. The abluminal stent area (white line) was delineated by connecting the abluminal strut surfaces with a trace line. In case the lumen contour was located within the abluminal stent area, struts were considered apposed, unless interrupted by a side branch (panel A/A’ 5 o’clock). Malapposed struts were defined as struts separated from the lumen contour (panel B). In panel B’, the lumen contour behind the malapposed struts at 11 o’clock is highlighted with a blue trace. In the presence of apposed struts that protruded into the lumen (panel C/C’) and where the lumen contour (dotted white line, panel D’) extended beyond the abluminal stent trace (continuous white line), a help line (blue line) was drawn in extension to the abluminal stent trace, and extrapolated between the lumen contour at these points (panels C’/D’). Struts were considered protruding (asterisks, panels C’/D’) only when the abluminal strut surfaces were separated from this help line, ie. when they protruded into the lumen more than the actual strut thickness.
The objectives of this study were (1) to analyse the relationship between the type of strut apposition at follow-up and clinical and procedural characteristics at stent implantation, and (2) to examine strut coverage as a surrogate for stent healing, in relation to strut apposition at follow-up. We hypothesised that protruding struts from a biological point of view have more similarity with malapposed struts than with apposed struts.
Methods
Patient population and lesion characteristics at stent implantation
Between September 2007 and September 2008, 35 patients were examined with OCT after stent implantation at our institution. Those with high quality OCT acquisitions, defined as an adequate visualisation of >70% of the vessel wall circumference throughout the stented area, were selected for this study. Patients with LST were excluded.
The morphology of each lesion at baseline was described from the coronary angiogram. Based on the hypothesis that certain lesion characteristics at baseline may influence strut apposition in the short- and/or long-term, we registered the following lesion characteristics: calcification, chronic total occlusion (>3 months), thrombus, recent total occlusion (<3 months), diffuse disease (>2 cm), excessive tortuosity and angulation >90º.11 As minor dissections, not necessarily visible on the angiogram, occur in connection with most coronary balloon dilatations12, we considered angiographically visible dissections as “significant”13, and hypothesised that these could influence the healing of the vessel wall behind the stent and hence have an impact on stent strut apposition. Angiograms were analysed independently by two experienced operators who were blinded to the clinical presentation. In the case of disagreement, a consensus diagnosis was obtained by repeated readings.
OCT image acquisition
OCT images were acquired with the M2 OCT system (LightLab Imaging Inc., Westford, MA, USA). Dependent on vessel anatomy, we used occlusive or non-occlusive techniques to displace blood during OCT imaging. After crossing the stent with an angioplasty wire, an over-the-wire OCT occlusion balloon catheter (Helios™) or a Renegade™ catheter (Boston Scientific, Natick, MA, USA) was advanced distally to the stented segment, and the coronary wire was exchanged with the OCT ImageWire™. For the occlusive method, the balloon catheter was retracted proximally to the stented segment and the balloon was inflated at 0.5 to 0.7 atmospheres during flush. For the non-occlusive method, the Renegade catheter was retracted into the guide catheter. During image acquisition, coronary blood was displaced by manual infusion of saline or Visipaque (Iodixanol 320; GE Health Care, Buckinghamshire, UK) for the occlusive and non-occlusive setup, respectively. Cross-sectional images were acquired at 15.4 frames/s, during automated pullback at 1 or 2 mm/s. Image acquisition was performed under haemodynamic monitoring. Although the non-occlusive technique may imply a slightly higher intracoronary pressure than the occlusive technique, the potential changes in the dimensions of the vessel wall and stent are likely to be proportional. We therefore assumed that the choice of technique would not influence strut apposition.
OCT image analysis
Cross-sections within the stented segment were analysed every 1mm by an experienced observer, who was blinded to the clinical and angiographic characteristics. Due to an underestimation of the actual thickness of the metal strut by the OCT infrared light, all strut thicknesses were reconstructed using a method described recently9. Based on apposition, struts were divided into four classes: (I) apposed struts; (II) struts overlying the ostium of a side branch; (III) malapposed struts; and (IV) protruding struts, as recently described (Figure1).9 The material covering struts was used as a surrogate for stent healing, and the minimum thickness was measured manually for every strut.
Statistical analysis
Statistical analysis was performed with the SPSS software, version 15.0 (SPSS Inc., Chicago, IL, USA). Normality of the quantitative data was assessed by visual estimation of residual plots. Continuous data are presented by their median and interquartile range (IQR), and categorical variables as percentages. Medians and frequencies were compared with the Mann-Whitney U-test and Fisher’s exact test, respectively.
We planned to compare (i) the presence or absence of strut coverage, and (ii) the thickness of strut coverage (TSC), between different strut appositional classes after pooling struts in four groups and adjusting for confounders related to stent healing, namely: time from stent implantation to follow-up14,15, clinical presentation at implantation16-18, diabetes mellitus19,20, stent type21,22, lesion type17,18, periprocedural characteristics, strut thickness and stent design.23 Due to the complex hierarchical structure of our data (stent struts nested within frames nested within lesions), the regression analysis requires a multilevel model. However, the distribution of TSC could not be transformed to normality, and a non-parametric equivalent that can adjust for relevant covariates, is not available. Instead, median TSCs for the various strut appositional types within every separate lesion with malapposed and/or protruding struts were calculated, and the differences were compared with a Wilcoxon signed rank test, paired at the lesion level.
For the comparison of frequencies of uncovered struts between appositional types, a logistic multilevel regression analysis was not possible due to the complex data structure, combined with a relatively high number of covariates and a heterogeneous study population. However, we present the frequencies of uncovered struts per strut appositional type. Details regarding struts overlying side branch ostia are also shown, but since these are pathophysiologically different from other “non-apposed” struts (malapposed and/or protruding struts), they were excluded from the analysis.
Intra-observer reproducibility of the classification of strut apposition and frequency of uncovered struts was assessed by calculating the Kappa coefficient. A two-tailed p-value ≤0.05 was considered statistically significant for all analyses.
Results
Strut apposition at follow-up OCT in relation to characteristics at stent implantation
Twenty-eight lesions (32 stents) from 28 patients were analysed. Eight lesions were located in the left anterior descending artery, six in the left circumflex artery, and 14 in the right coronary artery. The stents examined were both DES: Cypher (43%), Taxus Liberté (21%), Endeavor Sprint (18%), and Taxus Express; and BMS: Multilink Vision, Costar, Micro-Driver, and Palmaz-Schatz (3.6% each). A total of 507 frames containing 5,159 struts were analysed. There were 4,907 apposed struts, 20 struts overlying side branch ostia, 16 malapposed struts, and 216 protruding struts. Since malapposed and protruding struts often occurred in the same lesions, and could therefore not be analysed separately, lesions were divided into two groups: those without (group 1, n=20), and those with (group 2, n=8) the presence of malapposed and protruding struts. All lesions from group 2 contained protruding struts, while four lesions had malapposed struts as well. The time from stent implantation to examination with OCT was similar (median (IQR): 18 (9 to 34) months and 18 (12 to 31) months for group 1 and 2, respectively, (p=0.78)).
The clinical and procedural characteristics at the time of stent implantation are shown in Table 1. There were no differences in patient characteristics and angiographic lesion characteristics before intervention between the two groups. None of the lesions involved bifurcations. Patients with lesions displaying malapposed and/or protruding struts at OCT follow-up, had a significantly higher frequency of acute coronary syndrome (ACS) at stent implantation, compared with patients without these types of strut apposition (75% vs. 25%, respectively, p=0.03).
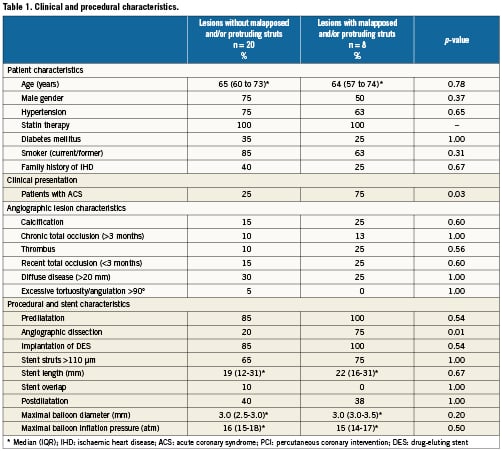
Lesions with malapposed and/or protruding struts at OCT follow-up were more often associated with angiographic dissections at baseline than lesions with apposed struts only (75% vs. 20%, p=0.01). Nine of these dissections occurred after predilatation and were all angiographically sealed by stent implantation, while one; appearing during stent implantation (type A), was considered benign without necessitating additional stenting. The location of dissections induced by predilatation before stenting coincided with the location of malapposed and protruding struts by OCT at follow-up (distal/mid/proximal one third of the stent). The dissections were of the following types: group 1: type A (n=1), type B (n=2), and type D (n=1); group 2: type A (n=1), type B (n=3), and type D (n=2).
Strut coverage in relation to strut apposition
Table 2 shows the frequencies of uncovered struts and the TSC per appositional type within the two groups of lesions. Without consideration of known confounders, non-apposed struts were more frequently uncovered, and had a tendency to have thinner strut coverage, compared to apposed struts. By paired analysis, considering the lesion from which different struts originate, there was a significant difference in the TSC of apposed compared to protruding struts (median difference (IQR): 50 (13 to 88) μm, p=0.018). A corresponding comparison between apposed and malapposed struts was not possible due to a small number of malapposed struts. Comparison of the TSC over different strut appositional types within individual lesions of group 2 shows a similar trend towards thinner strut coverage over malapposed and protruding struts (Table 3).
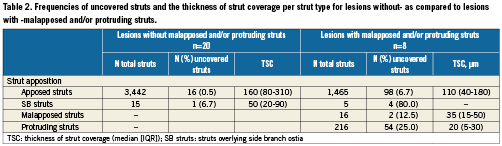
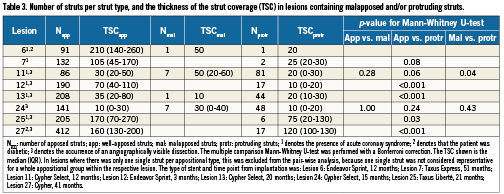
The intra-observer agreement was very good for the classification of strut apposition (Kappa coefficient [95% CI] = 0.94 [0.91 to 0.96]), and good for the identification of strut coverage (Kappa coefficient [95% CI] = 0.78 [0.75 to 0.81].
Clinical outcomes
Immediately after the follow-up OCT examination, two of the assessed lesions were treated for restenosis and one for malapposed struts. Additionally, six patients were treated for another lesion. LST did not occur in any patient by a median (IQR) follow-up time of 26 (24-27) months after OCT examination. During this time period, 20 of 27 patients (one patient died from a non-cardiac cause four months after OCT examination) received double antiplatelet therapy and one patient received clopidogrel therapy alone. The indications for use of clopidogrel were: OCT follow-up <12 months after DES implantation (n=3), new interventions (n=14), myocardial infarction without stent implantation (n=1), malapposed- and uncovered struts (n=1), previous LST (n=1), and aspirin intolerance (n=1).
Discussion
The main findings of the present study are: (a) Malapposed and protruding struts at OCT follow-up were more frequently associated with ACS and angiographically visible dissections at stent implantation, than lesions without these strut types. Further, (b) malapposed and protruding struts seemed more often uncovered, and tended to have thinner strut coverage than apposed struts, suggesting that strut apposition may be related to stent healing. These results support the hypothesis that protruding struts resemble malapposed struts more than apposed struts. We therefore find it relevant to identify protruding struts on OCT, in order to further investigate their clinical importance.
EVALUATION AND IMPORTANCE OF STRUT APPOSITION FOR STENT HEALING
One of the aims of coronary stent imaging with OCT is to study stent healing in vivo in order to evaluate the risk of LST. A histopathological study has previously shown that a lack of endothelial coverage over stent struts was a typical finding in cases of LST.10 The resolution of OCT is, however, insufficient to detect endothelial cells, which have a thickness of <10 μm. The histological study further suggested that a high ratio of uncovered struts per cross-section may be a morphometric predictor of LST.
As defined histologically, “uncovered” struts are struts that lack coverage of neointimal tissue components (extracellular matrix, smooth muscle cells and endothelial cells). Struts covered with fibrin (per definition not “tissue”) are thus “uncovered”.24 Although strut coverage can have various appearances by OCT, it is not fully known whether OCT can actually distinguish a neointimal layer from fibrin, although recent findings suggest that this might be possible with a new optical frequency domain imaging system.25 The true ratio of uncovered struts per cross-section might therefore be underestimated with OCT. Further, it should be noted that the histological ratio of uncovered struts per section, as used to estimate an odds ratio for LST, was based on an average of five cross-sections per stent.10 Factors that may influence the size of the ratio of uncovered struts per cross-section, and thus the determination of a cut-off value indicating the risk of LST, include: (1) the number of struts per cross-section, which depends on the stent design (in our study ranging from four to 23, median 10 struts/cross-section); (2) the average number of cross-sections included in the analysis (OCT can provide up to several hundreds of cross-sections per stent); and (3) the method of selection of cross-sections (in our study, selected cross-sections were equally spaced every 1 mm of the stent up to 33 cross-sections per stent, whereas by histopathology, typically 3-5 cross-sections per stent are selected from the proximal, mid, and distal segment). Quantitative analysis of strut coverage with OCT should therefore be interpreted with caution.
The present study showed a tendency towards less pronounced strut coverage over malapposed and protruding struts, as compared to apposed struts. Although OCT and histology has been used to evaluate apposition and coverage of intracoronary stents in several studies, a specific analysis of the degree of strut coverage in relation to apposition does not seem to have been performed previously.4,17,18,26-33 As described by pathology, stent healing follows a distinct pattern, beginning with the deposition of fibrin around struts. The fibrin is infiltrated by inflammatory cells, which subsequently attract smooth muscle cells from the media. These produce a proteoglycan and collagen-rich matrix, which constitutes the main component of the neointima.14 When struts are separated from the vessel wall, smooth muscle cells may not be able to “reach out” to them, which might explain why non-apposed struts, in the present study, were more frequently uncovered and had thinner coverage than apposed struts. Together with the antiproliferative effects of DES, the lack of apposition to the vessel wall may retain these struts in an early healing phase.
Clinical and angiographic lesion characteristics at stent implantation
Our present findings suggest that ACS and major dissections may predispose to malapposed and protruding struts at follow-up. Several reports have already proposed a link between late stent malapposition and ACS17,18,34,35, however, this has not previously been shown for protruding struts. Since baseline OCT examinations were not available in our study, it is not possible to evaluate whether malapposed and protruding struts were acquired or persistent. In the context of ACS, possible mechanisms could be: (a) for persistent malapposition: inappropriate apposition of struts to the vessel wall due to the presence of thrombi or necrotic cores in lipid plaques; (b) for (acquired) protrusion: inappropriate apposition and baseline with subsequent fibrin deposition between struts and the vessel wall due to blood turbulence or as part of the initiation phase of the healing process (Figure 2A); or (c) for acquired malapposition: dissolution of thrombus after stent implantation.2
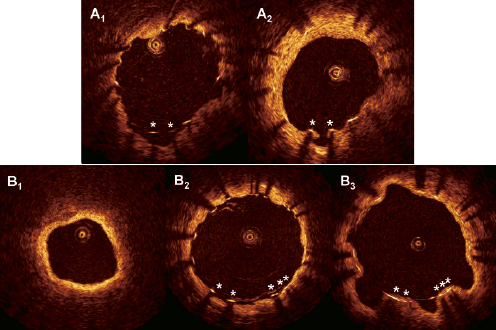
Figure 2. Evolution of protruding struts. The cross-sections come from two patients (A and B) with protruding struts at follow-up (A2 and B3) where abaseline evaluation was available. Panels A1 and B2 show the matched cross-sections immediately after stent implantation. Theprotruding struts (asterisks) in A2 were malapposed at baseline (panel A1), possibly with subsequent fibrin deposition and/or neointimal healing. Asopposed to this, the protruding struts in panel B3 were apposed to a regular lumen contour (panel B2) after implantation, without any evidence of thrombus in the examination before stent implantation (panel B1). The lumen contour thus seemed to have undergone positive remodelling at follow-up. Stent areas were similar at abseline and follow-up.
Despite the higher frequency of ACS at stent implantation in patients with malapposed and protruding struts at OCT examination, it was not accompanied by a difference in the angiographic presence of thrombus. This may be explained by the fact that smaller thrombi may be difficult to detect angiographically, or concealed in total occlusions.
In the context of procedural dissections as predisposing factors for malapposed and protruding struts, possible mechanisms could involve: (a) incomplete apposition of the stent to the vessel wall in areas of dissections (for persistent malapposition); (b) as (a) but followed by fibrin deposition between struts and the vessel wall (for acquired protruding struts); or (c) for acquired malapposed struts: a positive vessel remodelling due to inflammation caused by a deeper penetration of the drug into the vessel wall as a consequence of the dissection, causing the vessel wall to retract from the stent with time, with a simultaneous thinning of the coverage (Figure2B). Protruding struts may in the latter case represent a “transition phase” of apposed struts during the vessel remodelling before they become malapposed. Ultrasonic reports have previously suggested expansive remodelling as a cause of late stent malapposition2,5,36,37. However, the resolution of IVUS is probably insufficient to display protruding struts, because the distance between the average abluminal strut surface and the help line between the pits in the lumen contour is approximately 100µm9, which is the resolution of IVUS.
The frequency of malapposition compared to previous studies
The frequency of OCT-detected malapposed struts varies considerably in previous reports, reflecting the heterogeneity of different studies. Out of eight recent OCT studie, only four reported the number of stents with malapposed struts.17,18,26-30,33 Takano and colleagues found that all 31 Cypher stents evaluated exhibited at least one malapposed strut at three months after implantation. At a median of nine months following implantation, Gonzalo et al describe that 75% of lesions in patients with ST-elevation myocardial infarction and 25% of lesions in patients with unstable (UAP) or stable angina (SAP) had at least one malapposed strut after implantation of four different DES. Similarly, Kubo et al found by serial examination of 55 patients (24 UAP/31 SAP) immediately after implantation of Cypher stents and at nine months follow-up, that malapposed struts were more frequent in the UAP compared to SAP group. In addition, the rate of lesions with at least one malapposed strut decreased from baseline to follow-up (67% vs. 32%, and 33% vs. 4% for UAP and SAP, respectively). In the present study, 14% of lesions displayed malapposed struts and 29% of lesions malapposed and/or protruding struts at a median of 18 months follow-up, which is in line with the findings of Kubo et al. In a recent report, Guagliumi et al described a type of “protruding” struts, which were more frequent in DES compared to BMS.33 These struts differ, however, methodologically from the ones described here, as we only included in this category struts that protruded into the lumen more than the actual strut thickness. Using a similar definition, emerging data from the SIRTAX-LATE trial suggest that 38% of lesions with first-generation DES display these struts at 5-year follow-up.38 Nevertheless, the rate of malapposed/protruding struts for different stent types, in different clinical settings and at different time points, needs further clarification in larger studies.
Clinical importance of MALAPPOSED AND PROTRUDING struts
We observed no occurrence of stent thrombosis at late follow-up after OCT examination. However, 74% of our patients were prescribed dual antiplatelet therapy during this time. In a recent meta-analysis, late stent malapposition evaluated by IVUS was associated with LST.39 Cook et al found stent malapposition in 77% of patients with LST, as opposed to 12% of patients routinely examined at eight months following stent implantation without this complication at two years.3 Out of the 13 patients with LST, only one was treated with both aspirin and clopidogrel at the time of the event (no report of the clopidogrel use in the control group). Several case reports describe the occurrence of LST after discontinuation of antiplatelet therapy, with or without angiographic or ultrasonic signs of malapposition. Due to the insufficient resolution of IVUS, the extent of strut coverage in relation to stent apposition has not been possible to evaluate adequately in these studies. It remains to be settled whether OCT-detected strut malapposition and protrusion together with evaluation of the degree of strut coverage, may be of additional value for predicting LST.
Limitations
Limitations of this study include the lack of a reference OCT examination at the time of the index procedure, and thereby the inability to differentiate between persistent and acquired malapposition. Moreover, the patient population was small and heterogeneous: stent implantation included both BMS and DES, and OCT follow-up was performed at different time points. Further, it cannot be excluded that strut apposition might have changed during the extended follow-up, and that this, together with the prolonged clopidogrel therapy could have influenced the natural clinical course of the studied stents.
Conclusion
Clinical characteristics at stent implantation such as ACS and major periprocedural dissections seem to influence strut apposition, which in turn may play a role in stent healing. The present study supports the concept that protruding struts may have more in common with malapposed than with well apposed struts. The incidence, as well as the pathophysiology and clinical importance of protruding struts, remains to be settled.
Conflict of interest statement
The authors have no conflict of interest to declare.

