Abstract
Aims: At present there exists no direct comparative data for the detection of in-stent tissue coverage as assessed by intravascular ultrasound (IVUS) and optical coherence tomography (OCT) in clinical settings. To explore this subject, we investigated the correlation between the IVUS and OCT measurements derived from a contemporary population.
Methods and results: The present study includes 20 patients who had stents imaged at a six months follow-up with both IVUS and OCT, acquired with an automated pull-back. Off-line analyses were done by an independent validated Core-Lab (RHR, Rome, Italy). Measurements of stent length obtained by IVUS and OCT were 16.3±3.0 mm and 16.2±3.8 mm respectively (p=0.82) and were similar to nominal length (16.3±3.3 mm). Luminal area in the OCT image set was lower than that obtained in the corresponding IVUS image set (3.83±1.60 mm2 vs 4.05±1.44 mm2, p<0.001), while stent area was significantly higher when measured by OCT (6.61±1.39 mm2 vs 6.17±1.07 mm2, p<0.001). The percentage of tissue coverage measured by IVUS was lower than that measured in the corresponding OCT image sets (35.5±16.4% vs 43.4±16.1%, p<0.001). Correlation coefficients were high for repeated OCT measurements by two different observers (r=0.99).
Conclusions: OCT can quantify in-stent coverage and detect strut healing with high reproducibility. IVUS tends to underestimate the percentage of in-stent tissue coverage as compared to OCT.
Introduction
Intravascular ultrasound (IVUS) is the current standard invasive intravascular imaging modality for the assessment of in-stent tissue coverage after stent implantation1,2. However, certain drawbacks of IVUS, particularly with regards to its limited definition of vessel micro-structure on a size scale 150-200 µm, are well described3. Recently, optical coherence tomography (OCT), due to its resolution in the range of 10-15 microns, has emerged as a sensitive tool to assess the presence of stent strut coverage4, which has a protective role against in-stent thrombosis and can’t be studied by IVUS. Furthermore, OCT enables the follow-up quantification of tissue growth or hyperplasia5. This can be obtained with higher accuracy than IVUS, as OCT promises to identify the inner stent struts contour and the interface between lumen and tissue coverage with high accuracy6. For these reasons, OCT could become the reference method to study surrogate endpoints of the commercially available stents, possibly providing evidence to guide the duration of antiplatelet treatment7.
Previous studies reported the correlation between OCT and histology in an ex vivo setting9,10 and a recent study evaluated the correlation between in vivo measurements by OCT and IVUS in animal models6. However, there are presently no data comparing IVUS and OCT in clinical settings for the quantification of vessel lumen and assessment of stent coverage (vessel healing). To explore this subject, we investigated the correlation between IVUS and OCT measurements derived from a contemporary population.
Methods
Patient population
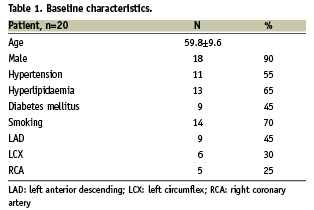
The present study includes patients who had stents imaged at a six month follow-up with both IVUS and OCT, acquired with an automated pull-back. A first group is made of 10 consecutive patients (10 stents) enrolled in the ATLANTA trial11, addressing the follow-up results of cobalt-chromium stents coated with Polyzene-F; a second group comprises 10 patients (10 cobalt-chromium stents) who entered our internal OCT registry (Table 1). The study was conducted according to the Declaration of Helsinki. The local medical ethics committee approved the protocol and written informed consent was obtained from every patient.
IVUS image acquisition and analysis
The IVUS images were obtained with mechanical ultrasound imaging catheters at 40 MHz (Atlantis 2.9 Fr, Boston Scientific, Natick, MA, USA) after intracoronary administration of 200 µg of nitroglycerin to prevent possible vasospasm. The imaging probe was positioned distally to the target lesions and withdrawn at a constant speed of 0.5 mm/sec using a motorised pull-back device. Off-line analyses were done with the EchoPlaque™ software (INDEC Medical Systems, Santa Clara, CA, USA). Lumen, stent, and external elastic membrane contours were analysed at a distance of 0.5 mm in the stented segment. The following measurements were obtained: mean stent area (SA), lumen area (LA), and percentage of tissue coverage area (%TCA). This latter was computed as ([SA-LA]x100/SA). Reproducibility of IVUS measurements from our core lab has been already reported12,13.
OCT imaging system and analysis
OCT images were obtained with a recently developed non-occlusive technique. Full details on this methodology are described elsewhere14,15. Briefly, once the image wire was positioned in the target vessel, pull-back was performed during simultaneous manual infusion of a commercially available viscous isosmolar contrast (Iodinoxanol 320, Visipaque™, GE Healthcare, Ireland) from the guiding catheter at an infusion rate between 1 and 3 cc/sec, based on the run-off of the artery and the online assessment of OCT image quality. By using this technique the blood is completely displaced from the artery during the whole acquisition. The OCT system used in this study consisted of an interface unit (Model M2 Cardiology Interface System, LightLab Imaging, Inc., Westford, MA, USA) providing images at a longitudinal resolution of 15 µm, and a 0.019-inch wire-type imaging catheter (ImageWire, LightLab Imaging, Inc., Westford, MA, USA) which contains a 0.006-inch fibre-optic imaging core and a distal radiopaque tip, like any other conventional guidewires. A motorised pull-back system at 2.0 mm/s was used, and OCT images were acquired at 15.6 frames/second.
All OCT frames were digitally stored and independently analysed in a validated core laboratory (Rome Heart Research, Italy). OCT images were deemed of good quality if they allowed accurate assessment along the whole circumference and led to appropriate measurements of stent and lumen area16. Only OCT pull-backs that enable an appropriate visualisation of the stented segment (with at least 90% of cross-section being of good quality) entered the study. As accurate calibration is mandatory to fully realise the spatial resolution of OCT, in all cases the size of the OCT image was calibrated prior to off-line analysis and monitored throughout the longitudinal segment, by adjusting the z-offset, the zero-point setting of the system.
SA and LA inside all stent struts were measured by manual trace at 0.5 mm intervals (every 4 frames). TCA was calculated as the difference between SA and LA. In line with the IVUS analysis, percent TCA was calculated as ([SA-LA]x100/SA). Measured tissue thickness >0 µm was defined as coverage.
As both IVUS and OCT acquisitions were done applying a continuous pull-back, and all OCT cross sections were analysed, it was possible to match any IVUS frame with the corresponding OCT one, using stent edges as anatomical landmarks. IVUS segments were matched to the OCT images with the percentile method on the basis of the total number of cuts by measuring the distance from the proximal end of the stent, as previously reported by Suzuki et al6.
Statistical analysis
Quantitative data were presented as mean±standard deviations, and were compared using Student t test. The correlation between IVUS and OCT was analysed by simple linear regression with 95% confidence intervals. Agreement was established by the Bland-Altman test. Inter-observer reproducibility was calculated for quantitative assessment by determining the mean and standard deviation of the between-observer differences and by providing linear correlation. For categorical data, the results were compared using the K-test of concordance. A two-sided p value of less than 0.05 was considered to indicate statistical significance. All data were processed using the Statistical Package for Social Sciences, version 15 (SPSS, Chicago, IL, USA).
Results
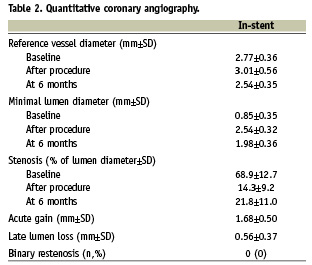
Stents had a nominal length comprised between 15 and 20 mm. In all cases an optimal result was obtained with a residual diameter stenosis less than 30% as assessed by quantitative coronary analysis. Table 2 shows the procedural and follow-up results. No major complications and/or arrhythmias were recorded during OCT acquisition.
Measurements of stent length obtained by IVUS and OCT were 16.3±3.0 mm and 16.2±3.8 mm respectively (p=0.82) and were similar to nominal length (16.3±3.3 mm).
There was a mild significant correlation for luminal area between IVUS and OCT (y=0.86+0.37x, r=0.37, p<0.001) and a clear correlation for stent area (y=0.95+0.76x; r=0.73; p<0.001) (Figures 1-2). However, luminal area in the OCT image set was lower than that obtained in the corresponding IVUS image set (absolute difference 0.22 mm2, relative difference –5.4%), while stent area was significantly higher (absolute difference 0.44 mm2, relative difference +7.1%) (Table 3). As a consequence, %TCA measured by IVUS was lower than that measured by OCT (absolute difference 7.9%, relative difference –22.3%). Even if a significant correlation between the two methods was obtained for %TCA (y=21±0.64x; r=0.65; p<0.001), the Bland-Altman analysis indicated that the 95% limits of agreement between the two methods when assessing %TCA ranged from –18.7% to 34.6% (Figure 3). Therefore, the two methods did not consistently provide similar measures because there was a level of disagreement that included clinically important discrepancies of up to 60%.
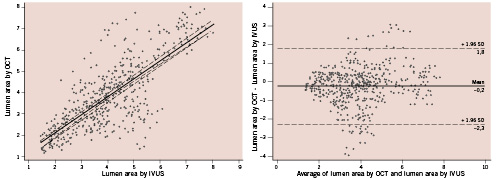
Figure 1. Comparison of the lumen area evaluated by optical coherence tomography (OCT) and intravascular ultrasound (IVUS) (left) and the Bland-Altman test for OCT vs. IVUS in the measurement of lumen area (right).
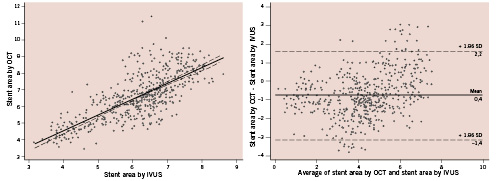
Figure 2. Comparison of the stent area evaluated by optical coherence tomography (OCT) and intravascular ultrasound (IVUS) (left) and the Bland-Altman test for OCT vs. IVUS in the measurement of stent area (right).
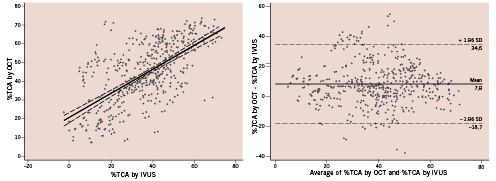
Figure 3. Comparison of the percentage of neointimal hyperplasia (%TCA) evaluated by optical coherence tomography (OCT) and intravascular ultrasound (IVUS) (left) and the Bland-Altman test for OCT vs. IVUS in the measurement of %TCA (right). Differences between lower and upper 95% limits outline discrepancies up to 60% among OCT and IVUS measurements.
The inter-observer differences were low for measurements of lumen area, stent area and %TCA by OCT (0.01±0.08 mm2, –0.05±0.20 mm2, –0.63±2.63% respectively). Correlation coefficients were high for repeated measurements by two different observers (r=0.99 for all measurements, p<0.001).
Presence of stent strut coverage was addressed in all stents (630 cross-sections, 4,442 struts). Absence of visible tissue coverage with OCT was found in 2.3% of struts and inter-observer test of concordance was 0.88 (p<0.001).
Discussion
OCT is a novel imaging technique that, due to its superb resolution in the range of 10-15 microns, is well suited for the identification of in-stent neointima. This methodology promises to have widespread use in the quantification of neointima and detection of vessel healing. The ability to detect even small degrees of tissue covering stent struts is of the utmost importance, as a lack of drug eluting stent coverage at follow-up is considered a major cause of late thrombosis17.
The main goal of this study was to validate the ability of OCT to address late in-stent restenosis, calculated as percentage of tissue growth, as compared with IVUS.
Our data suggest a distinct correlation between OCT and IVUS data, currently used as the gold standard, even if IVUS showed higher values of lumen area and lower values of stent area.
There are some possible explanations for this finding. Previous studies pointed at two main reasons: 1) the vessel stretching induced by IVUS Dotter effect18 and 2), the luminal reduction caused by the pressure drop, related to the occlusive modality of acquisition. However, these two factors cannot be called into question to explain these discrepancies, as arterial occlusion during the OCT study was avoided and lumen areas were in all case larger than the IVUS catheter. It is likely that the main reason for the discrepancies of lumen area measurements is in the higher resolution of OCT, as compared to IVUS, that leads to a sharp visualisation of the interface lumen-neointima19. Recently, Gonzalo et al reported in vivo OCT and IVUS comparison of lumen area assessment in human coronary arteries of patients not undergoing stenting20. Consistent with our own findings in patients undergoing PCI, they observed that IVUS tends to overestimate the lumen area compared to OCT.
With regards to measurements of stent areas, again, the better resolution of OCT enables a more accurate outline of the stent and OCT readers tend to trace the contour on the struts and not the inside. The fact that OCT leads to underestimation of the lumen area and an overestimation of the stent area has an impact on the measurement of percentage of tissue coverage, that obviously is greater by OCT as compared with IVUS. This was confirmed by Bland-Altman analysis and corroborates the findings of Suzuki et al10 obtained in animal models. These authors showed that OCT detects higher degrees of in-stent tissue growth than IVUS.
A second message provided by the present paper is that OCT assessment of strut coverage is highly reproducible. This is a key issue, as OCT has now a widespread use to address vessel healing after stenting.
In the near future, the possibility of combining accurate qualitative assessment of in-stent strut coverage and quantitative measurements of in-stent tissue growth, promises to make OCT the ideal technique to study stents at follow-up.
Movement during the heart cycle tends to affect the position of intracoronary catheters such as IVUS and OCT. In fact, catheters are displaced a bit backward and forward during acquisition, possibly affecting the accurate detection of length measurements of imaged segments hampering matching of corresponding IVUS and OCT cross-sections. Interestingly, cardiac cycle movements were found to have a trivial impact on the segment length, as stent length measured by IVUS and OCT were similar.
Limitations
This is a study dealing with a small, selected population with some degrees of neointimal hyperplasia. The major benefit of OCT is in the detection of strut coverage at micron levels, a concept not entirely linked to neointimal hyperplasia. Another caveat is that we did not use histology as the gold standard for comparison, since this study was designed as an in vivo comparison of IVUS vs OCT.
The comparison in a per frame fashion of two techniques with a very different lateral resolution, such as IVUS and OCT, is difficult and may be affected by some limitations. However, we did not assume this issue to significantly affect the reliability of the comparisons among frames, as matching was performed carefully using appropriate landmarks and checked for quality.
The incomplete visualisation of the whole circumference of lumen-plaque boundary is currently a draw-back of OCT and may occur when the OCT image wire has a non-coaxial location in large coronary segments. This problem that will be solved with the adoption of the second generation of OCT (Frequency Domain), but it never occurred in the consecutive series of stents that entered the present study.
Conclusions
OCT can quantify in-stent tissue growth and detect tissue strut coverage with high reproducibility. IVUS tends to underestimate percentage of in-stent tissue coverage as compared to OCT.

