Abstract
Aims: A novel balloon delivery system (BDS) for the self-apposing STENTYS® sirolimus-eluting stent (SES) has been developed for highly precise longitudinal stent positioning and deployment. The aim of this first-in-man study is to report the quantitative coronary analysis (QCA) angiography and optical coherence tomography (OCT) results as well as the 30-day clinical outcomes of the STENTYS Xposition S SES.
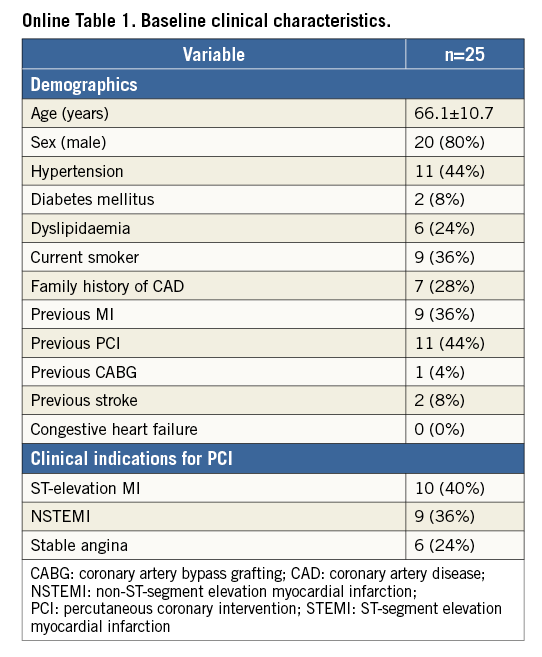
Methods and results: We included 25 patients (mean age 66.1±10.7 years) with stable coronary artery disease (24%) or acute coronary syndrome (including STEMI in 40%). The device was successfully placed at the intended site in all cases (100%), without procedural complications. Longitudinal geographic miss (entire lesion length [on QCA] not completely covered by the stent) was not observed. Pre-procedural MLD on QCA was 1.30±0.74 mm and post-procedural MLD was 2.74±0.44 mm, p<0.001 (acute gain 1.44±0.70 mm). OCT analyses showed a low percentage of malapposed stent struts directly post stent placement (2.4%), which further decreased after post-dilatation (0.6%, p=0.013), while mean stent area increased (from 9.7 mm2 to 10.5 mm2, p<0.001). At 30-day clinical follow-up, one (4%) major adverse cardiac event (MACE) was observed. One acute stent thrombosis (ST) occurred immediately post procedure in a STEMI patient which was related to inadequate medication therapy.
Conclusions: This first-in-man study demonstrated that the use of the novel STENTYS® Xposition S balloon delivery system is feasible with a high technical success rate without longitudinal geographical miss. Stent strut malapposition rate directly after STENTYS placement was low and improved further after post-dilatation.
Introduction
The concept of self-expanding stents in interventional cardiology was initially abandoned after the first-in-human WALLSTENT™ (Boston Scientific, Marlborough, MA, USA) study due to the chronic vessel wall injury caused by a relatively high chronic outward force1. However, newer generations of self-expanding stents made of nitinol, including the self-apposing STENTYS® stent (STENTYS S.A., Paris, France), with a more gentle chronic outward force might overcome this limitation and prove to be useful in anatomies in which proper stent apposition is challenging, such as ST-segment elevation myocardial infarction (STEMI)2,3 or (left main) bifurcations in which there is a large difference in the proximal and distal diameter4,5. However, optimal positioning might be challenging due to shortening of the stent as deployment is performed by retracting an outer sheath which releases the stent from the distal edge. A novel balloon delivery system (BDS) for the STENTYS sirolimus-eluting stent (SES), the STENTYS® Xposition S (STENTYS S.A.), was therefore developed to improve accurate stent positioning. We performed a first-in-man study of this new system assessing acute results, using post-procedural quantitative coronary angiography (QCA) and optical coherence tomography (OCT), and performing a 30-day clinical follow-up.
Methods
The SETUP study (Study to evaluate the safety and feasibility of the STENTYS Balloon Delivery System) was a prospective, single-arm first-in-man study performed in two Dutch centres (Academic Medical Center, Amsterdam, The Netherlands, and Albert Schweitzer Hospital, Dordrecht, The Netherlands) to evaluate the feasibility of the STENTYS Xposition S in de novo coronary lesions. Patients planned to undergo PCI of native coronary arteries with a reference vessel diameter between 2.5 and 6.0 mm and a lesion length <25 mm were eligible for enrolment. There were minimal exclusion criteria (Online Appendix). All patients provided written informed consent prior to the procedure and the study was approved by the local ethics committee. Patients were clinically evaluated at 30 days post procedure. QCA analysis was performed pre- and post-procedure (Online Appendix). OCT pullbacks were performed directly after STENTYS placement and after final post-dilatations using the C7-XR™ OCT intravascular imaging system (St. Jude Medical, St. Paul, MN, USA) with the C7 Dragonfly™ OCT catheter (St. Jude Medical), pullback speed 20 mm/s; 100 frames/s (Online Appendix). QCA and OCT analyses were conducted at the Academic Medical Center, Amsterdam, The Netherlands.
DEVICE DESCRIPTION AND PROCEDURE
The self-apposing STENTYS® Xposition S is pre-mounted on a novel balloon delivery system, which was not CE-marked at the time of the study (STENTYS SES is CE-marked and commercially available). The stent is made of nitinol (nickel/titanium alloy) and is available in three lengths (17, 22 and 27 mm) and three sizes suitable for reference vessel diameters of 2.5-3.0 mm (small), 3.0-3.5 mm (medium) and 3.5-4.5 mm (large), compatible with a maximum vessel diameter of 6 mm. Strut thickness is 103 microns (small size) or 133 microns (medium and large sizes). The novel delivery system, which is 6 Fr compatible, consists of the stent folded on a delivery balloon, which is covered with a distal “splittable” 0.0032” thick sheath assembly. The nominal diameter of the delivery balloon is the same as the smallest diameter for which the stent is suitable. When the semi-compliant delivery balloon is inflated within the sheath using low pressures starting at 8 atmospheres (atm), it causes the sheath assembly to split, allowing the STENTYS stent to deploy in the coronary artery at the desired location (Figure 1). The sheath is then withdrawn with the balloon. Lesion preparation, including thrombus aspiration and predilatation, is at the discretion of the operator. Balloon post-dilatation after STENTYS placement is strongly recommended and was done with OCT guidance2.

Figure 1. The novel delivery technique for the STENTYS Self-Apposing stent. The stent is mounted on a semi-compliant balloon and is restrained by a splittable sheath (A). Balloon inflation splits the sheath and releases the stent (B). The balloon is then deflated leaving the sheath between the stent and the vessel wall (C). The balloon and sheath are withdrawn leaving the stent well apposed to the vessel wall (D).
OUTCOME DEFINITIONS
Technical success was defined as the ability to cross and deploy the stent at the intended site. Angiographic success was defined as residual stenosis <30% and post-PCI Thrombolysis In Myocardial Infarction (TIMI) 3 flow on visual estimation. Longitudinal geographic miss (GM) was defined as the entire length of the stenotic segment (as defined by QCA) not completely covered by the stent (Figure 2). Procedural success was defined as angiographic success without in-hospital major adverse cardiac events (MACE). MACE was defined as the composite of cardiac death, recurrent target vessel myocardial infarction (TV-MI), and clinically driven target lesion revascularisation (CD-TLR). Other endpoints included: death from any cause, cardiac death, any MI, any target vessel revascularisation (TVR), definite ST and probable ST according to the Academic Research Consortium (ARC) definitions6.
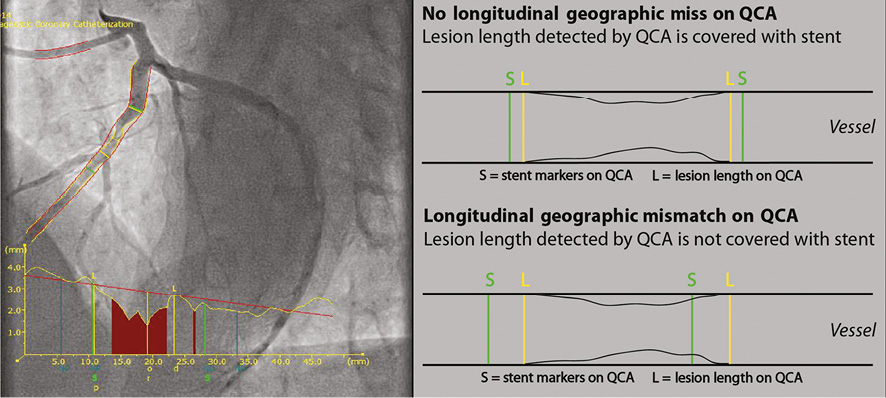
Figure 2. Longitudinal GM measurements using QCA analyses.
STATISTICAL METHODS
Continuous variables are presented as mean±standard deviation (SD), and categorical variables as frequencies (percentage). Comparison of OCT data post stenting with data post dilatation was evaluated with the Wilcoxon test. IBM SPSS Statistics for Windows software package, Version 21 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
BASELINE CLINICAL CHARACTERISTICS (Online Table 1)
A total of 25 patients were included (age 66.1±10.7 years, 80% male). Indication for PCI was STEMI in 40%, NSTEMI in 36%, and stable angina in 24%.
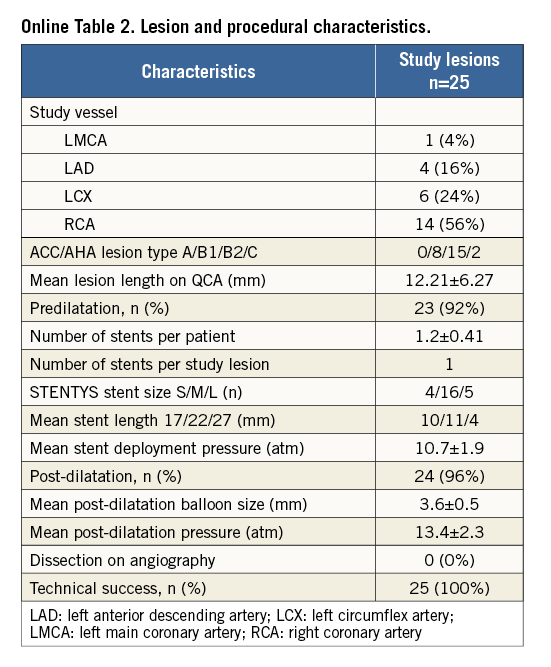
PROCEDURAL OUTCOME (Online Table 2)
Operators reported the handling characteristics of the device to be favourable. However, retraction of the sheath from behind the stent struts after deployment requires a gentle pull, taking care not to let the tip of the guiding catheter be advanced into the ostium of the vessel. Predilatation was performed in 23 cases (92%). Mean stent deployment pressure was 10.7±1.9 atm, and post dilatation was 13.4±2.3 atm. No post-procedure dissections were observed. Technical success was achieved in all patients (100%).
THIRTY-DAY CLINICAL OUTCOME
MACE occurred in one patient (4%). There was one recurrent non-fatal MI which was an acute stent thrombosis (ST) directly post procedure, treated with thrombus aspiration during repeat PCI. This patient initially received an inadequate periprocedural dose of heparin of 5,000 IU. There was one other major event not fulfilling the MACE definition: one patient experienced a non-fatal MI which was related to a non-target vessel treated with PCI. There were no other adverse events at 30-day follow-up.
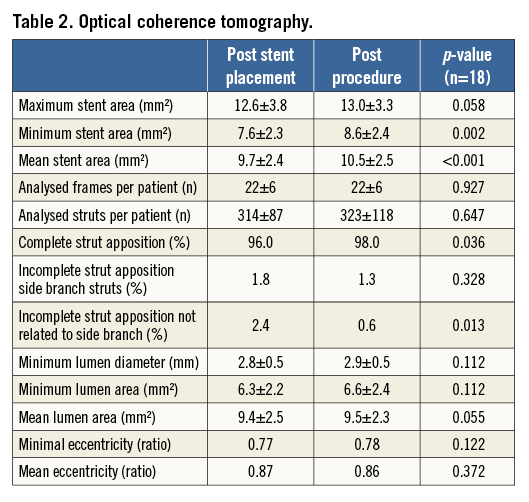
QCA AND OCT RESULTS (Table 1, Table 2)
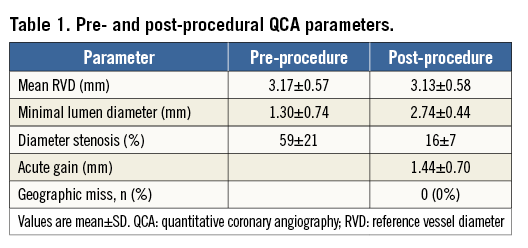
Pre-procedural MLD was 1.30±0.74 mm, and 2.74±0.44 mm after post-dilatation (acute gain 1.44±0.70 mm). Longitudinal GM was not observed (0%). The percentage of malapposed struts on OCT was significantly lower post procedure (0.6%) than directly post stent placement (2.4%, p=0.013). The mean stent area increased significantly from 9.7±2.4 mm2 post stent placement to 10.5±2.5 mm2 post procedure (p<0.001).
Discussion
This prospective, multicentre, first-in-man study evaluating the novel BDS for the STENTYS® SES, the STENTYS® Xposition S, showed that: 1) procedural success was 100% with real-world lesion complexities, including ostial lesions, highly calcified lesions and lesions with high thrombus burden; 2) there was no longitudinal GM as assessed with QCA; and 3) OCT data showed a high rate of acute stent strut apposition directly after stent deployment which improved further after post-dilatation.
Stent mis-sizing (resulting in axial GM) and mispositioning (resulting in longitudinal GM) during PCI are underappreciated, and found in more than 60% of cases7. Previous studies have shown that longitudinal and axial GM results in endothelial flow disturbances, increased intramural wall stress and increased wall shear stress, causing unfavourable healing with more pronounced intimal hyperplasia formation8. The STENTYS self-apposing stent platform offers a solution to the problem of axial GM by the self-apposing properties of the nitinol platform, as shown in STEMI patients in whom there is an increased risk of axial mismatch due to the presence of thrombus and vasoconstriction in the acute phase3,9. To overcome the risk of longitudinal GM, the novel STENTYS® Xposition S was developed for the self-apposing STENTYS SES. Indeed, we did not observe any longitudinal GM, although this needs to be confirmed in a larger sample size and by randomised comparison.
Study limitations
This study has several limitations. It was a small, non-randomised study with short-term clinical follow-up. The small number of patients does not allow any definite conclusions to be drawn concerning clinical outcomes. QCA and OCT analyses were not performed in the core lab of a clinical research organisation. However, we performed our QCA and OCT at an academic centre according to standardised methods using standardised standard operating procedures (SOPs). Larger sample sizes and randomised trials are warranted to evaluate the clinical outcome of this novel delivery device.
Conclusion
This first-in-man study demonstrated that the use of the novel delivery system for the STENTYS SES, the STENTYS® Xposition S, is feasible with a high technical and angiographic success rate without longitudinal geographic miss on QCA. Stent strut malapposition directly after STENTYS placement (as assessed with OCT) was low and was further improved by balloon post-dilatation.
| Impact on daily practice With this first-in-man study we have shown that the use of the novel delivery technique STENTYS® Xposition S in all-comer patients was feasible, with a high technical success rate. There was no longitudinal geographic miss and the final stent strut malapposition rate was low. The use of this device could be considered in anatomic subsets with a high risk of stent mispositioning and stent mis-sizing, such as lesions with a very high thrombotic burden, lesions located in ectatic/aneurysmal vessels, or bifurcation lesions with large differences between the proximal and distal diameters, lesions of the left main coronary artery, and ostial located lesions. Future randomised trials comparing the STENTYS self-apposing stent with conventional balloon-expandable stent devices are needed to prove the superiority in lesions with a high risk of stent mispositioning and mis-sizing. |
Funding
The authors all received research grants to their institutions from STENTYS S.A., Paris, France.
Conflict of interest statement
R. Spaargaren is an employee of STENTYS. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. The novel delivery system STENTYS Xposition S for accurate stent positioning and delivery.
Online Appendix. Inclusion and exclusion criteria SET-UP
INCLUSION CRITERIA
1. Age between 18 years and 80 years.
2. Patients with an indication to receive a STENTYS SES according to the IFU*.
3. Signed informed consent.
4. Reference vessel diameter >2.5 mm and <6.0 mm by visual assessment.
5. Target lesion ≤25 mm in length by visual estimate.
EXCLUSION CRITERIA
CLINICAL
1. Pregnant or breastfeeding woman or woman in fertile period not taking adequate contraceptives.
2. Currently enrolled in another investigational device or drug study that has not completed the primary endpoint or that clinically interferes with the current study endpoints.
3. Patients on anticoagulation therapy (coumadin).
4. Known intolerance to aspirin, clopidogrel, heparin, stainless steel, sirolimus, contrast material.
5. Known thrombocytopaenia (PLT <100,000/mm3).
6. Active bleeding or coagulopathy.
7. Cardiogenic shock.
8. Major planned surgery that requires discontinuation of dual antiplatelet therapy.
9. Comorbid condition(s) that could limit the subject’s ability to participate in the study or to comply with follow-up requirements, or impact on the scientific integrity of the study.
ANGIOGRAPHIC
10. Severe tortuous, calcified or angulated coronary anatomy of the study vessel.
11. Myocardial infarction due to stent thrombosis, or infarct lesion at a previously stented coronary artery.
12. Unprotected left main coronary artery stenosis >30% by visual assessment.
13. Staged procedure is planned within 30 days**.
14. Target lesion is a chronic total occlusion.
15. Perforated vessels evidenced by extravagation of contrast media.
*Inclusion of STEMI patients allowed if sufficient time available to demand written informed consent before stent placement.
**Treatment of other lesions during the same procedure in target and non-target vessel is allowed.
QCA ANALYSIS
Off-line QCA measurements using an end-diastolic frame in the same view selected by the interventional cardiologist pre- and post-stenting using validated software (QAngio XA, version 7.3; Medis medical imaging systems bv, Leiden, The Netherlands) were performed in-stent as well as in-segment (including the 5 mm proximal and distal to the stent). Longitudinal GM as assessed with QCA was defined as the entire length of the stenotic segment (as defined by the QCA software) not being completely covered by the stent. The MLD is automatically defined by the software. An interpolated reference diameter is automatically defined by the software. Diameter stenosis is calculated by subtracting the MLD from the interpolated RVD (at the MLD site) dividing by that same interpolated RVD value. QCA was performed in two projections, matched pre- and post-PCI, and the average of the analyses was taken.
OCT ANALYSIS
OCT measurements were obtained using validated software with a semi-automated contour and strut detection system (QIvus, version 7.3; Medis medical imaging systems bv, Leiden, The Netherlands). All cross-sectional images (frames) were initially screened and excluded from analysis if any portion of the image was out of the screen or the image had poor quality caused by residual blood or sew-up artefact10. Side branches occupying >45° of the cross-section were excluded from the analysis. Strut-level analysis was performed considering every ten frames (1 mm interval) along the entire target segment. Lumen and stent areas and volumes were calculated. Strut malapposition was determined when the distance from the endoluminal strut surface to the vessel wall was higher than the strut thickness plus 20 µm to correct for the blooming effect. The following OCT variables were collected: percentage of stent struts uncovered/malapposed, stent volumes, and lumen volumes. Malapposed struts were further subdivided into non-apposed side branch (NASB) struts and malapposed struts without side branch involvement.