
Abstract
Aims: Our aim was to assess the safety and efficacy of paclitaxel-eluting balloon (PTX-B) treatment after bare metal stent (BMS) implantation in patients undergoing primary angioplasty.
Methods and results: After BMS implantation, patients were randomised (1:1) to treatment with a PTX-B or no PTX-B treatment (BMS group). The primary endpoint was in-stent late luminal loss (LLL) at nine-month follow-up. OCT was carried out on the first 20% of consecutive patients included in the study. Two hundred and twenty-three patients were randomised (BMS: 112, PTX-B: 111). At nine months, median LLL was 0.80 mm (interquartile range [IQR] 0.36-1.26) in the BMS group vs. 0.31 mm (IQR 0.00-0.58) in the PTX-B group, p<0.0001. Binary restenosis was significantly lower in the PTX-B group: 29.8% vs. 2.2%, p<0.0001, 95% confidence interval (CI): 3.2-54.2. Nine-month OCT showed good strut coverage in both groups but greater in the BMS group (100±0.0% vs. 99.52±1.11%, p=0.03) with very low rates of malapposed struts per lesion. One-year MACE was significantly lower in the PTX-B group (12.5% vs. 3.6%, p=0.016).
Conclusions: PTX-B after successful BMS implantation resulted in less LLL and better clinical outcomes as compared with a BMS-only strategy. This was associated with good stent strut coverage and very low rates of malapposed struts. PEBSI trial (NCT 01839890)
Abbreviations
BMS: bare metal stent
DES: drug-eluting stent
EES: everolimus-eluting stent
LLL: late luminal loss
MACE: major adverse cardiac events
MLD: minimal lumen diameter
PTX-B: paclitaxel-eluting balloon
QCA: quantitative coronary angiography
ST: stent thrombosis
STEMI: ST-segment elevation myocardial infarction
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
In ST-segment elevation myocardial infarction (STEMI) primary angioplasty with stent implantation is currently the treatment of choice in most patients. Bare metal stents (BMS) have long been considered the benchmark for safety in STEMI due to the increased risk of late and very late stent thrombosis associated with first-generation drug-eluting stents (DES)1. Second-generation DES offer superior safety in STEMI2,3. However, caution is recommended, since there is still limited information regarding very long-term follow-up. Both clinical and histologic studies after DES implantation have demonstrated evidence of continuous neointimal growth during long-term follow-up (“late catch-up” phenomenon). Furthermore, in-stent neoatherosclerosis is an important substrate for late and very late stent failure for both BMS and DES4. We must therefore continue the quest for new devices and procedures, aiming for an improved safety/efficacy balance.
Paclitaxel-eluting balloons (PTX-Bs) have proven efficacy in many scenarios5, with an excellent safety profile. Theoretically, these devices achieve complete endothelialisation in less than three months. Additionally, the absence of a polymer coating prevents hypersensitivity reactions, a well-known contributor to late stent thrombosis. Furthermore, the entire surface of the PTX-B (not just the surface of the stent struts) is involved in delivering paclitaxel to the arterial wall. This produces a more homogeneous drug impregnation than DES, which could represent an additional biological advantage.
A combination of a BMS with the aforementioned advantages of a PTX-B might provide a benefit in the treatment of STEMI patients. Consequently, the purpose of our study was to compare the efficacy and safety of the combined treatment of a BMS plus a PTX-B with the conventional treatment (BMS only) during primary angioplasty in patients with STEMI.
Methods
The PEBSI study was a multicentre, single-blind, randomised controlled trial. The study was carried out according to the Declaration of Helsinki and approved by the local ethics committees of all participating centres. Signed informed consent was obtained from all patients included in the study. In addition to independent on-site monitoring, an independent clinical events committee, blinded to treatment allocation, adjudicated all clinical events. Finally, a data privacy monitoring board, independent and blinded to treatment allocation, evaluated the study regularly throughout the research period.
Patient population
PATIENT INCLUSION CRITERIA
Patients older than 18 years, in the first 12 hours after STEMI onset, and indicated for primary PCI were eligible. STEMI was defined by electrocardiographic ST elevation of at least 1 mm in two or more contiguous leads or new left bundle branch block, or a true posterior myocardial infarction with angiographic evidence of a single culprit lesion in the target vessel.
EXCLUSION CRITERIA
Clinical exclusion criteria included cardiogenic shock, life expectancy less than 12 months, and women of childbearing age. Procedural exclusion criteria included unprotected left main stenosis >50%, bifurcations with side branch greater than 2.5 mm, stent thrombosis, lesion length >30 mm (exceeding the longest available PTX-B), reference vessel diameter <2.5 mm or >4 mm, more than one severe stenosis in the same coronary artery, patients amenable to CABG within 30 days post STEMI, and overlapping stents used to treat the culprit segment.
DEVICES
The paclitaxel-eluting balloon used in this study was the Pantera Lux (Biotronik, Berlin, Germany), coated with a homogeneous dose of paclitaxel (3 mcg/mm²). The microcrystalline structure of paclitaxel is maintained using butyryl-trihexyl citrate (BTHC).
The BMS used in this study was the PRO-Kinetic Energy stent (Biotronik), a cobalt-chromium stent platform with a 60 μm strut thickness and double helix stent design.
Procedure
INTERVENTIONAL PROCEDURE
Vascular access (radial or femoral) was left to the interventionalist’s discretion. All patients received a pre-procedural loading dose of aspirin (250 to 500 mg) and clopidogrel (600 mg), prasugrel (60 mg), or ticagrelor (180 mg). Heparin (70-100 U/kg) was administered before the procedure. Use of glycoprotein IIb/IIIa inhibitors and additional boluses of heparin were left to operator discretion. Stent length was selected to cover the stenosis completely and stent size was selected to achieve a stent/distal artery ratio of 1-1.1/1. Pre- and post-dilation before randomisation was left to the operator’s discretion.
RANDOMISATION
After successful BMS implantation (final TIMI flow 2-3, final residual stenosis <30%, and no post-implantation complications), patients were randomised to one of two groups in a 1:1 ratio: PTX-B group (post-dilation with PTX-B for 45 seconds) and BMS group (no further post-dilation). Interventional treatment type was allocated using opaque sealed and sequentially numbered envelopes using a computer-generated randomisation code. In the PTX-B group, only one PTX-B was allowed (i.e., treatment of a 30 mm stent segment with two 15 mm PTX-Bs was not allowed) and only a single 45-second PTX-B inflation was allowed. The PTX-B diameter was selected to achieve a 1.1:1 ratio of the final BMS diameter according to the manufacturer’s pressure/diameter tables. The length of the PTX-B had to be equal to the length of the previously chosen stent, or slightly longer but taking care to avoid balloon protrusion more than 2 mm from each edge of the stent.
FOLLOW-UP
Patients were treated according to current clinical practice guidelines. Dual antiplatelet therapy was prescribed for 12 months. All patients were scheduled for a clinical follow-up at one, six, and 12 months post procedure and for a nine-month control angiography.
ENDPOINTS AND ADVERSE EVENTS
The primary endpoint was in-stent late luminal loss (LLL) as measured by quantitative coronary angiography (QCA) at nine-month follow-up.
SECONDARY ENDPOINTS
Target vessel revascularisation (TVR) and target lesion revascularisation (TLR) were defined according to ARC criteria6. Ischaemia-driven TLR and TVR were indicated if the patient presented with a >50% stenosis and angina, or objective signs of silent ischaemia such as via a stress test or an FFR <0.8. Reinfarction was defined according to the WHO extended definition. Major adverse cardiac events (MACE) were defined as a composite of death, non-fatal target vessel reinfarction, or ischaemia-driven TVR. Target vessel failure (TVF) was defined as a composite of cardiac death, reinfarction of the treated vessel, or ischaemia-driven TVR. Stent thrombosis was classified according to ARC criteria6. Bleeding was defined according to ARC criteria.
QUANTITATIVE CORONARY ANGIOGRAPHY
An independent core laboratory analysed all the angiograms using standardised instructions. QCA was performed using standard image quantification software (Medis, Leiden, The Netherlands). QCA personnel were not involved in the study and were blinded to randomisation assignments. Pre-procedural images were taken before thrombus aspiration. In post-procedural and follow-up images, the in-stent and the additional 5 mm segments proximal and distal to the stent edges (in-segment) were analysed. Minimal lumen diameter (MLD), lesion length, and reference vessel diameter were measured by QCA. Acute gain was defined as the difference between the post-procedural and pre-procedural MLD. Binary restenosis was defined as a diameter stenosis ≥50% at nine-month follow-up. LLL was defined as the difference between post-procedural MLD and nine-month follow-up MLD of the same segment.
OCT SUBSTUDY
OCT was performed in the first consecutive patients included in four centres (affiliations 1, 2, 3, 6 of the study’s authors), until 20% of the total sample was reached. The OCT imaging was obtained at nine-month follow-up and performed after intracoronary nitroglycerine injection, using the C7-XR™ Fourier-Domain System (St. Jude Medical, St. Paul, MN, USA). OCT data were analysed by an expert analyst, who was blinded to clinical presentation and lesion characteristics, using proprietary off-line software (St. Jude Medical). OCT analysis is described elsewhere7.
Statistical analysis
The primary endpoint of the study was in-stent LLL at nine-month follow-up angiography. Since at the time when the trial was designed there were no efficacy data published in STEMI with PTX-Bs, it was estimated that a sample size of 220 patients (assuming a 10% loss to achieve a minimum of 200 study participants) would be needed to detect a significant reduction of LLL of 0.8 mm in the BMS group compared with 0.4 mm in the PTX-B group, with a power of 80% and an alpha of 0.05. LLL estimates for this calculation were based on published data from clinical trials of BMS and DES in STEMI, assuming that the PTX-B would double the LLL reported for studies of DES in STEMI8.
Continuous variables are shown as a mean±SD or median plus interquartile range. Categorical variables are shown as counts and percentages. We used the Shapiro-Wilk test to analyse the normality of the distributions. Differences were analysed per protocol. Continuous variables were compared using the Student’s t-test for independent normally distributed data or the Wilcoxon test in the absence of normal distribution. Categorical variables were compared using the chi-square or Fisher’s exact test. The one-year event rates were estimated using Kaplan-Meier time-to-event methodology and were compared using the log-rank test. To confirm the stability of the observed differences between treatments, an adjustment of the primary endpoint (LLL) with its starting value as covariate (post-procedural MLD) was performed using the analysis of covariance model (ANCOVA). Before that, normal distribution of the outcome variable in each group was established. A two-tailed p-value <0.05 was considered statistically significant.
Results
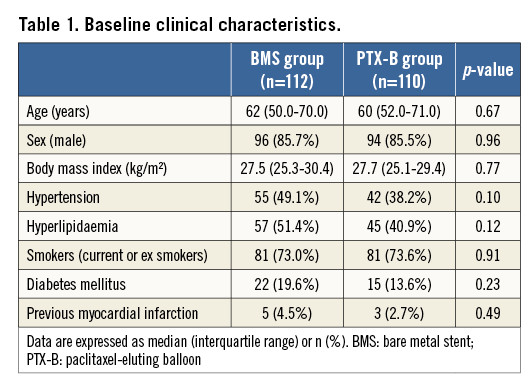
BASELINE CHARACTERISTICS AND PROCEDURAL DATA
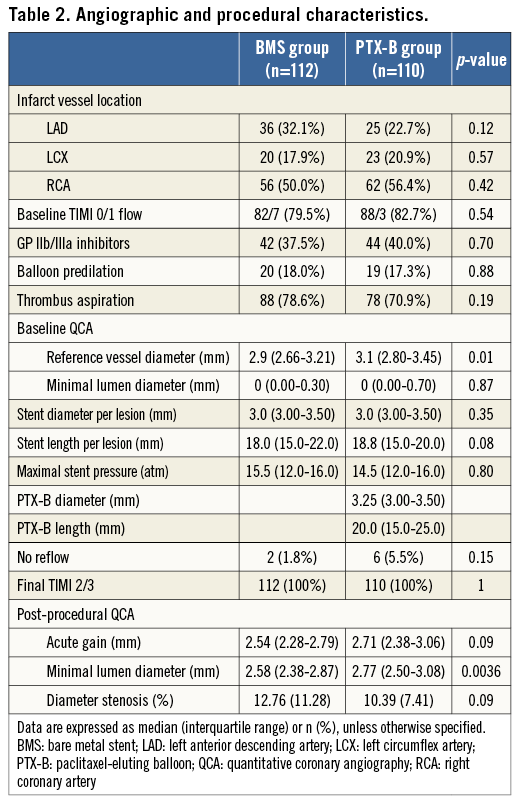
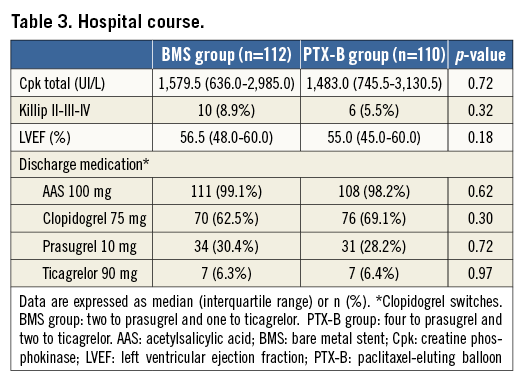
From April 2012 to July 2013, 223 patients were randomised (112 in the BMS group and 111 in the PTX-B group). The flow chart of the study is shown in Figure 1. Overall, clinical, angiographic and procedural characteristics and the hospital course were similar in both groups (Table 1-Table 3). Radial access was used in 195 patients (87.8%). All patients in the PTX-B group could be treated with the PTX-B. There was no need to use any additional interventional techniques to deliver the PTX to the target segment. Reference vessel diameter before intervention was higher in the PTX-B group (2.97±0.43 vs. 3.12±0.46 mm, p=0.01). Similarly, post-intervention MLD was higher in the PTX-B group (2.6±0.4 vs. 2.8±0.4 mm, p=0.004); however, acute gain was similar in both groups (2.54±0.46 vs. 2.67±0.59 mm, p=0.08). No patients in the BMS group were post-dilated after stent implantation.
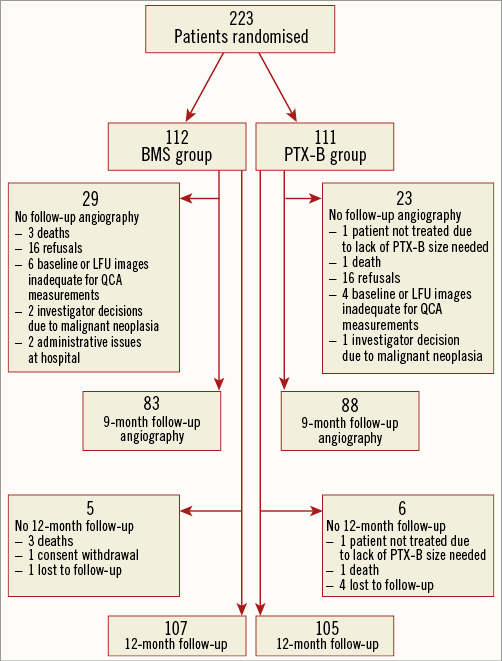
Figure 1. Flow chart of the study. BMS: bare metal stent; LFU: late follow-up; PTX-B: paclitaxel-eluting balloon; QCA: quantitative coronary angiography
ANGIOGRAPHIC FOLLOW-UP: PRIMARY ENDPOINT
Nine-month QCA data are shown in Table 4. The primary endpoint, in-stent LLL at nine-month follow-up angiography was a median of 0.80 mm (interquartile range [IQR] 0.36-1.26) in the BMS group vs. 0.31 mm (IQR 0.00-0.58) in the PTX-B group, p<0.0001. MLD at follow-up was higher in the PTX-B group: 1.79±0.71 mm vs. 2.48±0.57 mm, p<0.0001; 95% CI: 0.50-0.88. After adjustment by post-intervention MLD, differences at nine months continued to favour the PTX-B group: LLL 0.87 vs. 0.29 mm, p<0.0001; MLD 1.85 vs. 2.43 mm, p<0.0001; and binary restenosis 29.6 vs. 2.4%, p=0.0003. There was no edge restenosis in either group. One diffuse coronary aneurysm (≥10 mm) within the stent was seen in the BMS group and two in the PTX-B group (one focal and one diffuse). Patients with and without angiographic follow-up had similar clinical characteristics (data not shown). Cumulative frequency distribution curves of MLD and percent diameter stenosis at different time intervals are shown in Figure 2, and Figure 3, respectively.
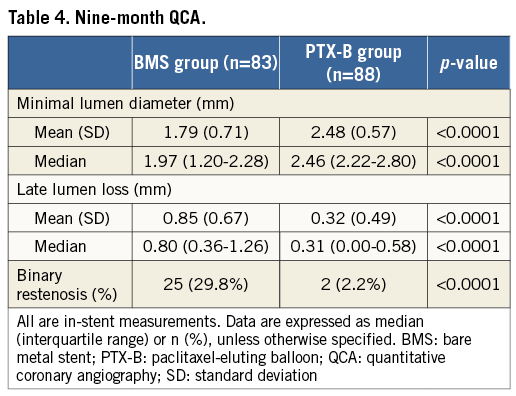
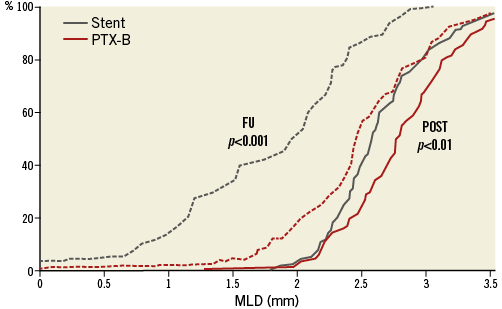
Figure 2. Cumulative frequency distribution curves of minimal lumen diameters. Minimal lumen diameters (MLD) are shown after the intervention (POST) (continuous line), and at late follow-up (LFU) (dashed lines).
Figure 3. Cumulative frequency distribution curves of diameter stenosis. Cumulative frequency distribution curves of the percentages of diameter stenosis after the intervention (POST) (continuous line), and at late follow-up (LFU) (dashed lines) are shown. The intersection of the dashed grey line (50%) with the distribution curves at LFU determined the binary restenosis (RE) rates in both arms.
OCT SUBSTUDY
Forty-four patients were included (BMS group: 19, PTX-B group: 25). A total of 8,782 struts were analysed (BMS: 3,776 struts, PTX-B: 5,006 struts). Strut coverage at nine months was good in both groups but greater in the BMS group (100±0.0% of struts covered vs. 99.52±1.11%, p=0.03). Uncovered struts in the PTX group were randomly distributed along the stent. The maximum uncovered strut length within the stent in the PTX-B group was 0.48±1.12 mm. The frequency of malapposed struts per lesion was very low in both groups (BMS: 0.36±1.00%, PTX-B: 1.35±3.03%, p=0.32). No clustering of incomplete strut apposition was found in either group. The maximum distance of the malapposed struts to the lumen was 0.05±0.14 mm in the BMS group and 0.14±0.27 mm in the PTX-B group, p=0.29.
ONE-YEAR CLINICAL FOLLOW-UP
One-year clinical events are shown in Table 5. MACE, TVF, and TVR were significantly lower in the PTX-B group (14 [12.5%] vs. four [3.6%], p=0.02; 13 [11.6%] vs. four [3.6%], p=0.03; and 10 [8.9%] vs. two [1.8%], p=0.02, respectively). In addition, there was a trend to a lower TLR in the PTX-B group: eight patients (7.1%) vs. two patients (1.8%) in the BMS group, p=0.056. Kaplan-Meier survival estimates for MACE and other individual components of the combined clinical endpoint at one-year follow-up are shown in Figure 4.
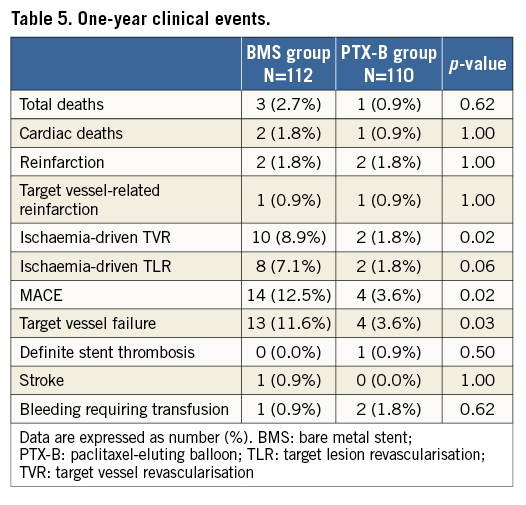
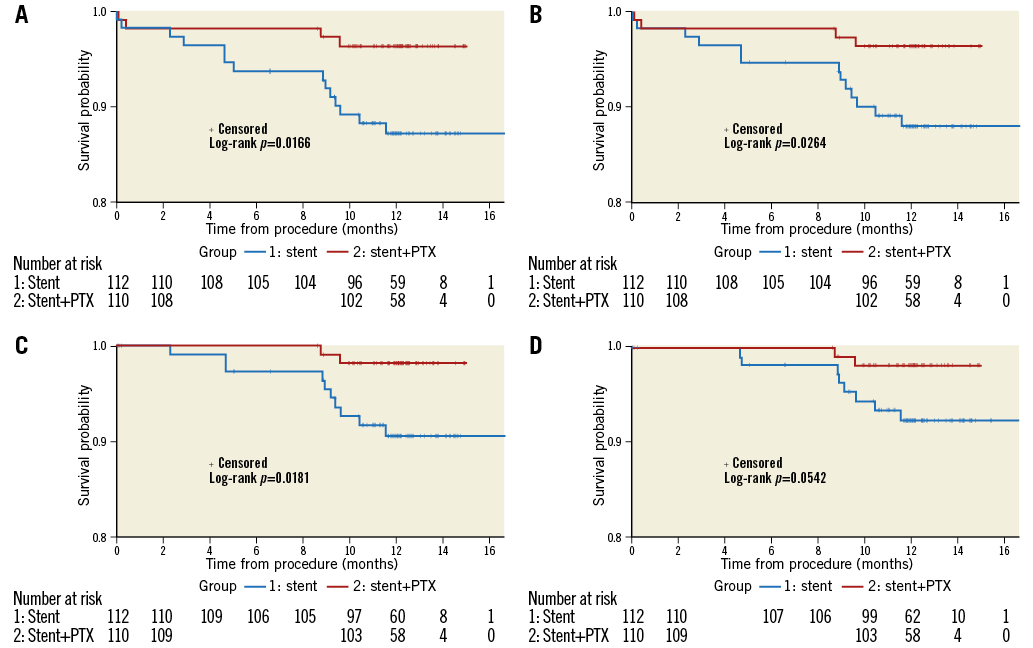
Figure 4. Kaplan-Meier cumulative incidence curves for clinical endpoints at one-year follow-up. A) MACE (a composite of death, non-fatal target vessel reinfarction, and ischaemia-driven TVR). B) Target vessel failure. C) Target vessel revascularisation. D) Target lesion revascularisation.
Discussion
The main finding of our study was that lesion impregnation with a paclitaxel-eluting balloon after BMS implantation was significantly more effective in reducing LLL than conventional treatment with BMS only. This resulted in a decreased rate of TVR and TVF in the PTX-B group. In addition, this efficacy was achieved without a significant compromise in strut coverage by OCT (99.5% of covered struts) and suggests better one-year clinical outcomes compared with BMS-only implantation in terms of MACE, TVF, and TVR, with very low rates of adverse safety outcomes.
This is the first study to show the efficacy of paclitaxel eluted from a balloon for the treatment of STEMI patients. Only two previous studies have been performed in this setting, both with negative results. The DEB-AMI trial was a single-arm study (presented at EuroPCR in 2011 but unpublished) that used the SeQuent® Please PTX-B (B. Braun, Melsungen, Germany). LLL was 0.48 mm at nine months, with unacceptably high rates of revascularisation (17%), and 6% vessel thrombosis at one year. The second trial, the DEB-AMI study9, used a second-generation DIOR® PTX-B (Eurocor GmbH, Bonn, Germany). Six-month angiographic data showed a 0.68 mm LLL and a binary restenosis rate of 24%, both similar to those obtained in the BMS-only group. In both of these studies, the procedural sequence was lesion preparation (thrombus aspiration, predilation), followed by impregnation of the target segment with paclitaxel released from a balloon, and finally BMS implantation. Our trial applied a completely different strategy, applying paclitaxel after stent implantation. There are four major reasons why this may explain the difference in our study results.
First, when paclitaxel is applied prior to stenting and directly on the plaque/thrombus, as in the two previously mentioned studies, it is released on an irregular plaque/thrombus and therefore the drug may be unevenly distributed. Areas with less plaque/thrombus lumen protrusion may receive less paclitaxel than anticipated because the PTX-B may not even be in contact with these areas. In addition, PTX-B inflation may fragment the thrombus and, when deflated, thrombus-containing paclitaxel would migrate distally, thereby losing efficacy at the target segment.
Second, applying the paclitaxel after successful stent implantation ensures fast, atraumatic delivery of the PTX-B to the target segment. A very quick and non-traumatic balloon transition to the target lesion appears to be central to the effectiveness of these devices.
Third, once the stent is implanted, accurate fluoroscopic recognition of the target segment is possible, thus reducing the risk of geographical miss, a major cause of failure of these devices. This is probably the reason why edge effects were not found in our study.
Fourth, the PTX-B chosen for this trial may have contributed to the results because not all PTX-Bs are equal10. They differ in the type of folding, antiproliferative drugs, coating technology, excipients, kinetics of elution, transference to the vessel wall, and durability of the drug in the coronary vessel. 2014 European guidelines on myocardial revascularisation clearly state that “one cannot assume a class effect for all drug-eluting balloons”. Balloons used in the two previous STEMI trials have a loading dose of 3 mcg paclitaxel/mm². However, the excipients differ. SeQuent Please uses iopromide and the second-generation DIOR balloon uses shellac. The excipient used in our trial was BTHC, a highly lipophilic compound that allows very quick, effective transfer of paclitaxel to the vessel wall.
At the end of the index procedure, the PTX-B group had a slightly larger MLD which could have had some impact on the final LLL; however, the considerable difference in LLL between both groups (0.85 vs. 0.32 mm) makes it very unlikely that this is the sole reason. Furthermore, after adjustment by post-intervention MLD, differences at nine months were even more favourable for the PTX-B group (0.87 vs. 0.29 mm).
Differences that favour the PTX-B over BMS in this study were not limited to angiographic efficacy. They were also driven by a reduction in ischaemic clinical endpoints. In the PTX-B group, one-year TLR was low (1.8%) and comparable to other studies of new-generation DES in STEMI, such as the COMFORTABLE AMI3 (TLR rate of 1.6%) or the EXAMINATION trial2 (TLR: 2.1%).
Regarding safety, the one-year stent thrombosis rate in our trial was low in the PTX-B group (0.9%). The only event occurred in a patient who discontinued all medications, including aspirin and clopidogrel. This low rate is comparable to that published in other trials for EES, currently the stent that seems to have the lowest rate of stent thrombosis (0.5% in the EXAMINATION trial2 and 1.2% in the XAMI trial11). Our safety results may be explained by the good strut coverage (99.5% of the struts covered) found by OCT in the PTX-B group along with the low percentage of malapposed struts. The absence of any polymer in the PTX-B technology, avoiding hypersensitivity reactions with the potential to cause stent thrombosis, might also have had a favourable impact on the results.
Further research is needed to determine whether the small amount of neointimal proliferation (0.3 mm neointimal layer) found in the PTX-B group, which is sufficient to cover the majority of the stent struts and limit stent malapposition, may represent a very long-term safety advantage of PTX-B protocols in STEMI, compared to second-generation DES. A patient-oriented trial with ischaemic clinical endpoints comparing PTX-B with second-generation DES will be available after the PEBSI II trial, which is currently underway. Until we have this information, we suggest using our strategy in cases of second-generation DES stent thrombosis where no obvious cause of thrombosis is proven.
Limitations
This study was powered for angiographic outcomes and did not aim to detect clinical differences between groups. Our study is only valid for those STEMI patients who have had a good angiographic result after BMS implantation. We do not know if the same efficacy would be found in other scenarios such as dissections, overlapping stents, bifurcations, etc. Despite the promising results of our study, longer follow-up is required to determine whether this favourable safety and efficacy profile is maintained over time, as continuing rates of late stent thrombosis have been reported in first-generation DES over at least five years, and late efficacy stent failure has been demonstrated for both BMS and DES, especially at long-term follow-up4.
Conclusions
Paclitaxel lesion impregnation from a balloon after successful BMS implantation shows angiographic superiority compared with a BMS-only strategy in patients with STEMI undergoing primary PCI. This efficacy is at the expense of achieving good strut coverage by OCT, with more than 99.5% of the struts covered, and a low percentage of malapposed struts. Finally, in addition to angiographic efficacy, differences in favour of the PTX-B over BMS in this study were driven by a one-year reduction in ischaemic clinical endpoints with very low rates of adverse safety outcomes.
| Impact on daily practice The results obtained in our study open up new lines of research on the percutaneous treatment of ischaemic heart disease using our strategy of “stent first” with the paclitaxel-eluting balloon technology used. Its effectiveness and security having been demonstrated, this also opens up new lines of investigation regarding dual antiplatelet therapy, since these devices might lead to a more rapid endothelialisation compared to drug-eluting stents. |
Acknowledgements
This study was promoted by FIC (Fundación Interhospitalaria para la Investigación Cardiovascular).
Funding
Funded by an unrestricted grant from Biotronik.
Conflict of interest statement
The authors have no conflicts of interest to declare.

