Abstract
Aims: The one-year outcome of lesions in small coronary arteries by using a paclitaxel-iopromide-coated (3 µg/mm²) balloon catheter (DCB) has yielded good six-month angiographic and one-year clinical data. We now report the three-year clinical follow-up.
Methods and results: One hundred and twenty patients with >70% stenoses <22 mm in length in small coronary vessels (vessel diameter: 2.25-2.8 mm) were treated with the DCB. The primary endpoint was angiographic in-segment late lumen loss. The secondary endpoints encompassed all other angiographic and clinical data up to three years post intervention. In total 82/120 (68.3%) patients with a vessel diameter of 2.35±0.19 mm were treated with the DCB only, and 32/120 (26.7%) patients required additional bare metal stent (BMS) deployment. Both the 12- and 36-month major adverse cardiac event rates were 5/82 (6.1%) for DCB only and 12/32 (37.5%) for DCB+BMS, primarily due to the need for target lesion revascularisation in 4/82 (4.9%) patients and 9/32 (28.1%) (p<0.001) patients, respectively. Total MACE rate after 36 months was 18/120 (15%; intention-to-treat).
Conclusions: Treatment of small vessel coronary artery disease with a paclitaxel-iopromide-coated balloon exhibited good six-month angiographic and one-year clinical data that persisted during the three-year follow-up period. Randomised trials will clarify its role as an alternative to drug-eluting stents in the treatment of small vessel coronary artery disease. (ClinicalTrials.gov Identifier: NCT00404144).
Introduction
Treatment of small coronary arteries by percutaneous transluminal coronary intervention represents about 35-40% of all procedures1. Long-term results are limited by a high recurrence rate. Uncoated coronary stents failed to reduce the rate of major adverse cardiac events compared to angioplasty alone2-4. Drug-eluting stents in small coronary vessels seem to be beneficial5,6, despite the fact that stent-based local drug delivery might be associated with delayed and incomplete endothelialisation and the potential risk of subacute and even late stent thrombosis7,8.
Randomised clinical trials have shown the efficacy of paclitaxel-iopromide-coated balloon catheters (DCB) in the treatment of bare metal and drug-eluting stent restenosis9-14, leading to a Class IIa recommendation for the treatment of bare metal stent restenosis in the 2010 European Guidelines for Revascularisation for this specific type of DCB15. Furthermore, randomised trials and large registries could demonstrate the potential clinical benefit in coronary de novo lesions16-19. However, randomised trials with other types of DCB have shown conflicting results in the treatment of de novo lesions in small coronary arteries20,21. In the Piccoleto trial the DIOR® balloon (Eurocor GmbH, Bonn, Germany) was inferior to the TAXUS® Liberté® stent (Boston Scientific, Natick, MA, USA) (57 patients with small coronary vessel disease) in terms of angiographic percent stenosis and a trend towards increased target lesion reinterventions20. Furthermore, the identical Elutax balloon (Aachen Resonance GmbH, Aachen, Germany) was significantly inferior to the paclitaxel-iopromide-coated balloon catheter (SeQuent® Please; B. Braun AG, Melsungen, Germany) in the SCAAR registry22. In contrast, in the BELLO trial (n=182) a paclitaxel-urea-coated balloon was non-inferior to the TAXUS® Liberté® stent in terms of late loss (primary endpoint) and also showed superiority21.
The aim of the PEPCAD I trial was to investigate the efficacy of a paclitaxel-iopromide-coated balloon catheter in the treatment of small vessel coronary artery disease. The present paper presents the three-year clinical follow-up results of this study population.
Methods
STUDY DESIGN
The study is a prospective non-randomised trial performed at eight German cardiology departments in Berlin, Dortmund, Jena, Rotenburg an der Fulda, Esslingen, Homburg/Saar, Heidelberg, and Halle. The study was sponsored by B. Braun Melsungen AG, Vascular Systems, Berlin, Germany, the manufacturer of the drug-coated balloon catheter. The sponsor had a role in the design of the study, but not in the analysis of the results, the decision to publish, or in the preparation of the manuscript. An independent CRO and core lab vouched for the accuracy and completeness of the data.
The study was performed according to the Declaration of Helsinki and World Health Organization guidelines. The requirements of sections 20 to 22 of the German Medical Device Law and of the European standard EN 540 were followed. Patients provided written informed consent prior to the procedure. The study was approved by the responsible local ethics committees.
Patients enrolled were at least 18 years of age with stable or unstable angina, or an abnormal functional study and a single de novo lesion in a native coronary artery with a reference diameter between 2.25 mm and 2.8 mm. Exclusion criteria comprised factors such as acute myocardial infarction within 48 hours preceding the procedure, severe renal insufficiency (GFR <30 ml/min), known hypersensitivity or contraindication to the required medication, and malignancies with a life expectancy of less than three years. Angiographic exclusion criteria encompassed lesions more than 22 mm long, stenoses below 70% of the luminal diameter, unprotected left main stenosis, lesions with a major side branch (>2 mm), and restenoses.
INTERVENTIONAL PROCEDURE
Patients received 250 mg of aspirin intravenously, heparin as an initial bolus of 70-200 IU/kg body weight adjusted according to the activated clotting time (200 sec to 250 sec), and clopidogrel as a loading dose of 300 mg the day before or 600 mg immediately before the intervention. Glycoprotein IIb/IIIa antagonists were administered at the operator’s discretion. After intracoronary injection of nitroglycerine (100 μg to 200 μg), baseline angiography of the target vessel was performed in at least two near-orthogonal views showing the target lesion free of foreshortening and vessel overlap. After assessment of the angiographic inclusion and exclusion criteria, each eligible patient’s target lesion was dilated once for at least 30 seconds with the paclitaxel-coated balloon catheter (SeQuent® Please; B. Braun Melsungen AG). The compliance of the balloon allowed for a diameter range from 2.3 mm (5 atm) to 2.8 mm (15 atm). In the case of severe elastic recoil or dissection, bare metal stents were deployed.
QUANTITATIVE CORONARY ANGIOGRAPHY
Angiography was performed before, during and after all interventions at six months and at ischaemia-driven unscheduled angiography using identical projections. Quantitative analysis of the coronary angiographic images was performed by an independent core laboratory (Clinical Research Institute, Rotenburg an der Fulda, Germany). The CAAS II system (Pie Medical Imaging, Maastricht, The Netherlands) was used for automated contour detection and quantification. Measurements included the stenotic area with measurement from shoulder to shoulder (in-lesion), and the total treated area plus 5 mm of the edges (in-segment). Restenosis was defined as a diameter stenosis of at least 50%.
FOLLOW-UP AND ENDPOINTS
All patients received ≥100 mg aspirin daily. Clopidogrel (75 mg/day) was given for one month following stand-alone drug-coated balloon angioplasty and for three months after additional bare metal stent implantation. Clinical follow-up was performed for three years. All endpoints and adverse events were adjudicated by an independent clinical events committee.
In-segment late lumen loss after six months was the primary endpoint. Secondary endpoints included the rate of restenosis and the rate of the combined clinical events up to three years, including stent thrombosis, target lesion revascularisation, myocardial infarction, and death. Stent thrombosis was defined according to the ARC definitions23. In this paper, the three-year clinical follow-up data are presented. Target lesion revascularisation was based on symptoms of coronary ischaemia, angiographic findings at scheduled or unscheduled follow-up, or both. Myocardial infarction was assumed to have occurred if two of the following five criteria were present: chest pain lasting longer than 30 minutes; electrocardiographic changes typical of acute myocardial infarction (ST-segment elevation of 0.1 mV in at least two adjacent ECG leads or new occurrence of a complete left bundle branch block); increase of creatine kinase concentration or its MB isoform to at least three times the upper normal value; new, clinically significant Q-waves; and chest pain leading to angiography up to six hours after the onset of the chest pain with angiographic evidence of an occluded vessel. Serious adverse events were defined according to international (ICH) guidelines.
STATISTICAL ANALYSIS
Data were analysed according to intention-to-treat. An as-treated analysis, e.g., comparing patients treated with the drug-coated balloon only and with additional stent implantation, was performed for descriptive comparison only. Continuous data are expressed as means±standard deviation. Categorical variables were compared with the chi-square test, continuous variables with the Student’s t-test or the Welch’s test for unequal variances. Confidence intervals for the difference between proportions were calculated with a normal approximation of the binomial distribution without correction for continuity (StatView 5.0 and BiAS 8.05). A two-sided p-value of <0.050 was considered to indicate statistical significance.
Results
PATIENTS
One hundred and twenty patients with a mean age of 68±8 years (72% men) were enrolled in the trial between January 2006 and December 2006. Baseline characteristics were typical for patients with diffuse and multivessel coronary artery disease, including 34% diabetic patients.
ANGIOPLASTY AND ANGIOGRAPHIC FOLLOW-UP
The mean vessel diameter was 2.36±0.19 mm with a lesion length of 11.5±4.7 mm. Two patients with severe violation of the protocol were excluded from further analysis (one each with a muscle bridge and an insignificant stenosis with no intervention performed). In four of the 118 patients (3.4%) the lesions could not be crossed by the study balloon (one B1 lesion and three B2 lesions). Two each of these four patients were treated with a conventional balloon catheter or with oral medication leaving 114 patients who were treated with the drug-coated balloon of 2.5 mm in diameter in each case. Eighty-two of the 120 patients (68.3%) were treated with the drug-coated balloon only, while in 32/118 patients (26.7%) elastic recoil (12 patients, 10.0%) or dissection (20 patients, 16.7%) required additional stent deployment (Table 1).
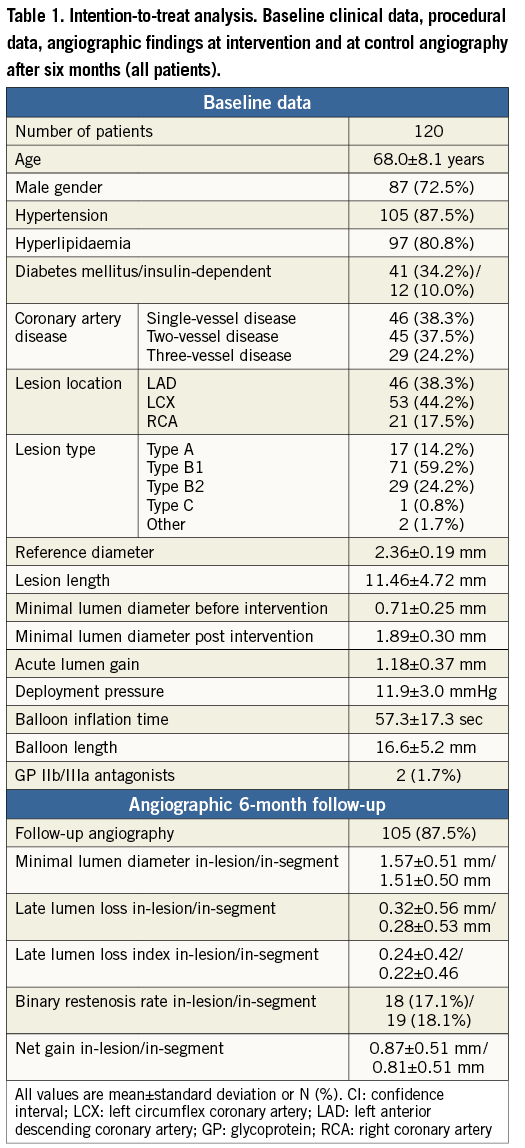
A total of 105/120 (87.5%) patients underwent follow-up angiography after 6.4±1.3 months, while 15/120 (12.5%) declined angiographic follow-up due to the absence of clinical symptoms. The mean in-segment late lumen loss was 0.28±0.53 mm. Restenosis occurred in 19/105 (18.1%) patients (Table 1).
CLINICAL FOLLOW-UP
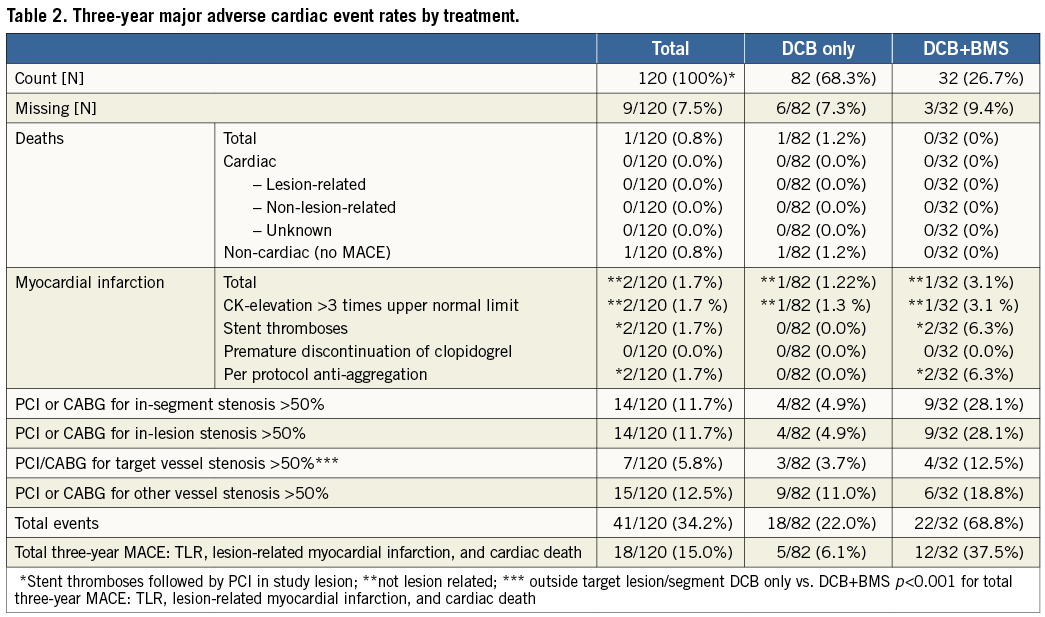
After three years, follow-up information was obtained in 111/120 (92.5%) patients, while 1/120 (0.8%) subject withdrew consent, and 8/120 (6.7%) patients were lost to follow-up (5/82 [6.1%] DCB only group, 3/32 [9.4%] additional BMS group). These patients lost to follow-up moved away and we have no patient consent to ask the registration office. In addition, the general practitioners were questioned without success. One patient died during this time from a non-cardiac cause in the drug-coated balloon-only group. Two non-ST-elevation myocardial infarctions occurred: one immediately post PCI in a patient treated with the drug-coated balloon only with no recurrence at scheduled six-month follow-up angiography, and one during the first year of follow-up in a patient who required a bare metal stent as part of the index procedure (Table 2). Another patient with additional bare metal stent implantation experienced a non-ST-elevation myocardial infarction, which was related to a high grade lesion in another vessel. Two patients with additional bare metal stent implantation presented with thrombotic occlusion of the stent two days and four months after the procedure, respectively. The patient with the subacute stent thrombosis was not under dual antiplatelet therapy. In both patients the initial procedure was complicated by geographical mismatch between the drug-coated balloon and the implanted bare metal stent. Fourteen patients (12%) underwent repeated target lesion revascularisation during the one-year follow-up period (Table 1).
ANALYSIS BY TREATMENT
In patients treated with the drug-coated balloon only, the in-segment late lumen loss was 0.16±0.38 mm (Table 1), and 0.62±0.73 mm in patients with additional bare metal stent implantation (p<0.001). Restenosis occurred in four of 73 patients (6%) in the drug-coated balloon-only group and in 13 of 29 patients (45%) in the group with additional bare metal stent implantation (p<0.001) (Table 3 and Table 4). In patients with additional bare metal stent implantation the total length of the stented area was a predictor for the occurrence of restenosis. In multivariate analysis geographical mismatch between the segments treated with the drug-coated balloon and the areas treated with an uncoated stent was identified as an independent predictor for the incidence of restenosis. In those patients with the occurrence of restenosis, the stent was longer than the drug-coated balloon (difference 2.3±10.7 mm), whereas in patients without restenosis the balloons were longer than the stents (difference 2.8±7.7 mm; p=0.10). The in-segment late lumen loss in patients with and without geographical mismatch was 1.00±0.74 mm and 0.32±0.59 mm, respectively (p=0.013).
The incidence of target lesion revascularisation was significantly different between patients treated with the drug-coated balloon only (4/82 patients, 5%) and those requiring additional bare metal stent implantation (9/32 patients, 28%, p<0.001) (Table 3). The difference in the total clinical event rate (6.1% with drug-coated balloon only vs. 37.5% with additional bare metal stent implantation, p<0.001) was mainly driven by target lesion revascularisation, primarily induced by geographical mismatch in patients with additional bare metal stent implantation. From one year to three years, 2/82 (2.4%) patients treated with the DCB only needed clinically driven target lesion revascularisation, whereas the same procedure was necessary in 1/32 (3.1%) of patients treated with the DCB and a BMS during the index PCI (Table 4). Hence, the total lesion-related rate of major adverse cardiac events after three years was 6.1% in patients treated with the drug-coated balloon only and 37.5% in those who needed a bare metal stent in addition (Figure 1).
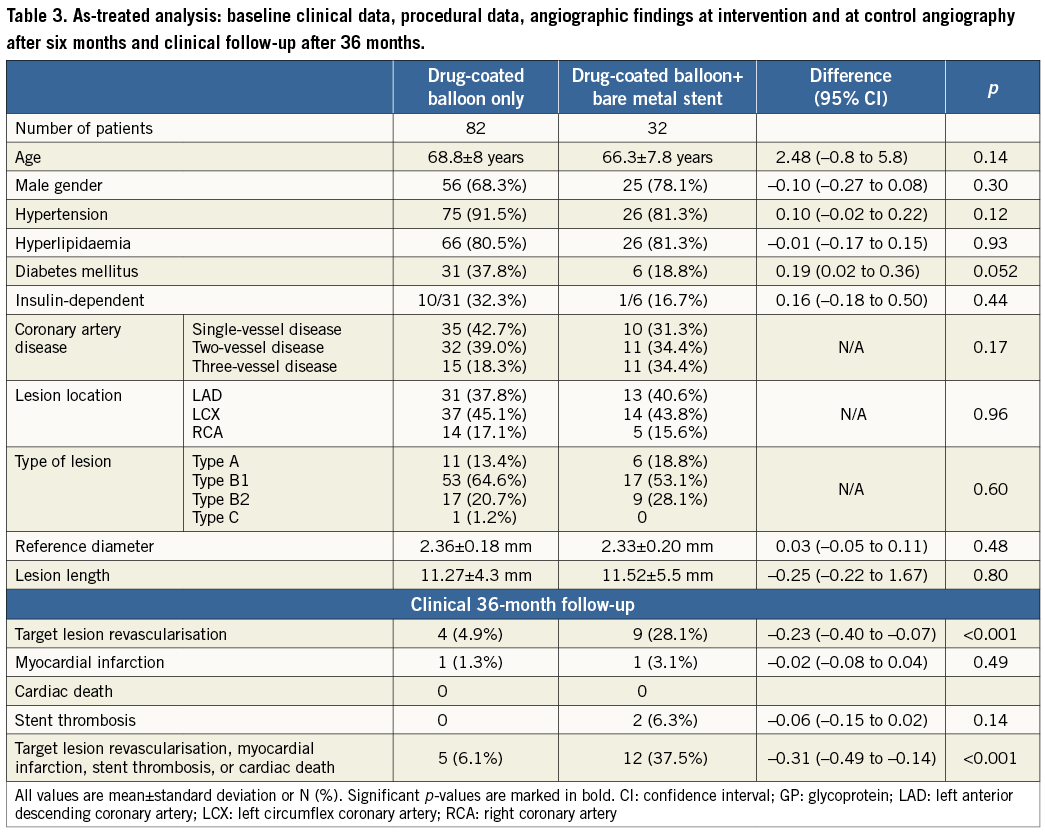
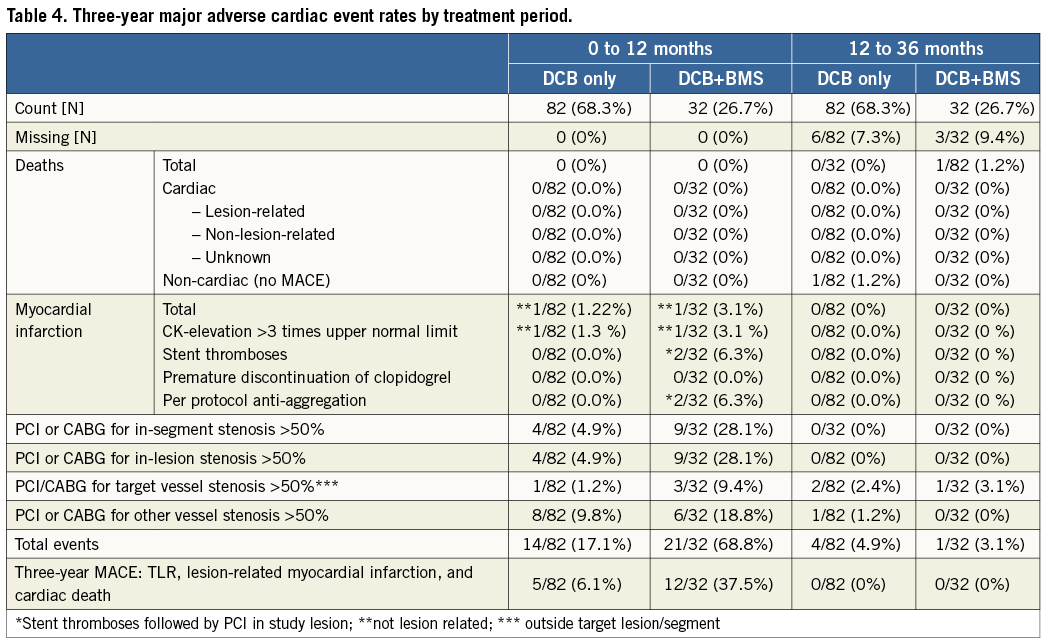
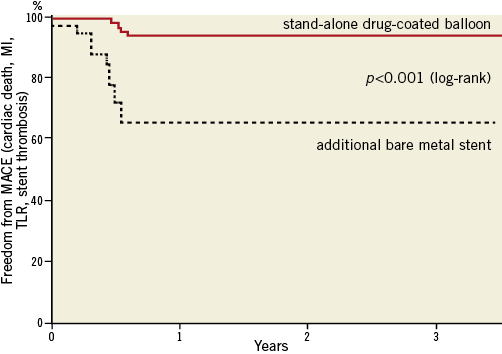
Figure 1. Freedom from MACE (cardiac death, MI, TLR, stent thrombosis).
Discussion
Vessel size represents a major determinant of the outcome after percutaneous coronary intervention. Vessel geometry in small coronary arteries limits compensation for neointimal proliferation following stent deployment. While conflicting data exist on the superiority of bare metal stent implantation over plain balloon angioplasty in this indication24-27, drug-eluting stents significantly reduce restenosis rates compared to uncoated stents28,29. However, even with drug-eluting stents, restenosis rates of up to 31% following the treatment of vessels with diameters of 2.8 mm or less have been reported30-32.
Paclitaxel-iopromide-coated angioplasty balloon catheters have shown reproducible efficacy in the treatment and prevention of restenosis in the porcine coronary model33-35, in patients with coronary in-stent restenosis up to five years post procedure11, and in human peripheral arteries36,37. The coating includes the x-ray contrast medium iopromide, which improves the solubility of the drug and its transfer to the vessel wall33,38.
In PEPCAD I SVD, the six-month angiographic and one-year clinical outcomes compared well with published data on drug-eluting stents in small coronary vessel disease5,28,30-32, as reported in detail earlier39. During one to three years of clinical follow-up, no major coronary event occurred in the present study. This holds true when the drug-coated balloon was used either as a stand-alone procedure or when followed by a bare metal stent deployment (Table 2).
Though the six-month occurrence of restenosis is higher when the drug-coated balloon catheter has to be combined with a bare metal stent for either dissection or pronounced elastic recoil, the findings of the present study suggest clinically stable lesions in both situations when the antiproliferative drug is delivered only during the short time of balloon inflation. On the other hand, when using drug-eluting stents the antiproliferative agent is released from the stent struts for a period of several weeks or months. This fact along with the possible local inflammation due to polymeric coatings might result in more pronounced and longer persisting endothelial dysfunction as shown in a porcine coronary model40. Moreover, endothelialisation is likely to be delayed or even missing with the ensuing consequences8,41. The clinical sequelae encompass late thrombotic events and the late catch-up phenomenon. For the latter, vessel size of below 2.5 mm has been reported to be an independent predictor42.
Dual antiplatelet therapy (DAPT) seems to be necessary for only one month after stand-alone procedures with the drug-coated balloon while current guidelines recommend twelve months of DAPT following drug-eluting stent deployment43.
Study limitations
The study is limited mainly by the fact that PEPCAD I is a single-arm study in a relatively small cohort of patients. Nonetheless, the present study is the third one that supports the continuous (at least three-year) clinical benefit of non-stent-based intravascular local drug delivery by paclitaxel-iopromide-coated angioplasty balloon catheters11. The treated lesions were clinically stable from six months onwards. Moreover, this is the first study that demonstrates three-year clinical safety and efficacy of this drug-coated balloon catheter in high risk de novo coronary artery lesions.
Conclusions
The study protocol did not provide specific rules for the use of additional bare metal stents. This may explain the high incidence of geographical mismatch in patients with additional stent implantation. Furthermore, only one balloon diameter (2.5 mm) was available, resulting in an increased risk for relevant dissections especially in the smallest vessels treated in this study. Based on the lessons learned from PEPCAD I, the so-called “DEB only” concept was developed44. The concept includes a careful predilatation with a balloon-to-vessel ratio of 0.8-1.0 to prepare the lesion and to assess the risk for a relevant dissection. It includes the use of non-compliant balloons and scoring balloons as well as additional imaging if helpful. In case of a relevant dissection (type C or higher), a residual stenosis of more than 30%, or a flow reduction, a conventional stent approach is recommended. Otherwise, the use of the DCB with nominal pressure will finish the procedure without stent implantation. In the BELLO study, the crossover rate to additional stent implantation was only 20%21. The SeQuent Please World Wide Registry confirmed the feasibility of the “DEB only” concept. In 559 de novo lesions 81% could be treated without additional stent implantation, resulting in a target lesion reintervention rate of only 1.0% after 9.8 months (2.4 % in patients with additional bare metal stent implantation)19.
Appendix
PEPCAD I STUDY GROUP
Principal Investigator: Martin Unverdorben, Clinical Research Institute, Center for Cardiovascular Diseases, Rotenburg an der Fulda, Germany; CRO and Angiographic Core Lab: Ralf Degenhardt, Clinical Research Institute, Center for Cardiovascular Diseases, Rotenburg an der Fulda, Germany. Staff: Tina Iffland, Melanie Häußler; Statistical Advisor: Hanns Ackermann, Center for Biomedical Informatics, Department for Biomathematics, University Frankfurt/Main, Frankfurt/Main, Germany; Internal Medicine, Unfallkrankenhaus Berlin, Charité University, Berlin, Germany: Franz X. Kleber, Sascha Rux, Daniel Grund; Heike Bull; 50 patients; Medical Clinic, Cardiology, St. Johannes Hospital, Dortmund, Germany: Hubertus Heuer, Norbert Schulze Waltrup, Joachim Weber-Albers, Maritta Marks, Axel Bünemann, Dietmar Schmitz, Mathias Stratmann; Martin Schulz, Claudia Rosendahl, Birgit Laschewski, Alexandra Thrun, Kathrin Euler, Ute Dieckheuer; 35 patients; Clinic for Internal Medicine, University Jena, Jena, Germany: Stefan Betge, Hans-Reiner Figulla; 13 patients; Cardiologic Clinik, Center for Cardiovascular Diseases, Rotenburg an der Fulda, Germany: Christian Vallbracht, Manfred Scholz, Henning Köhler, Bernd Abt, Eberhard Wagner; 7 patients; Clinik for Cardiology, Pneumology and Angiology, Esslingen, Germany: Matthias Leschke, Jean Rieber; Birgit Blaich; 8 patients; Clinic for Internal Medicine III, University of the Saarland, Homburg/Saar, Germany: Bruno Scheller, Bodo Cremers, Michael Kindermann, Michael Böhm; Nicole Hollinger, Bianca Werner; 4 patients; Internal Medicine III, University Heidelberg, Heidelberg, Germany: Helmut Kücherer, Stefan Hardt; Christiane Selter; 2 patients; Internal Medicine III, University Halle, Halle, Germany: Michael Buerke; Michaela König; 1 patient
Funding
The study was supported by B. Braun Melsungen AG, Germany.
Conflict of interest statement
B. Scheller reports being co-inventor of a patent application for various methods of restenosis inhibition, including the technique employed in this trial, by Charité University Hospital, Berlin. M. Unverdorben receives salaries from the Institut für Klinische Forschung, Herz- und Kreislaufzentrum, Rotenburg an der Fulda, Germany; during part of the study he was employed by B.Braun. F. Kleber and B. Cremers received lecture fees from B.Braun. M. Boxberger is an employee of B.Braun. The other authors have no conflicts of interest to declare.

