Introduction
Percutaneous coronary intervention is the most frequent revascularisation therapy in patients with significant coronary artery disease. However, the interventional revascularisation of small coronary arteries remains still challenging. Also there is no universally accepted definition of what constitutes a “small vessel”, though a vessel diameter of <2.8mm is usually considered as small vessel disease. PCI of small vessels is performed in more than 100,000 cases in Germany per year, and therefore constitutes a considerable proportion of interventional procedures. In the German ALKK registry, the rate of PCI in vessels <2.8mm is quite constant with 35-40% over the years (Figure 1). Therefore the results of PCI in small vessels are of high medical and economic importance.
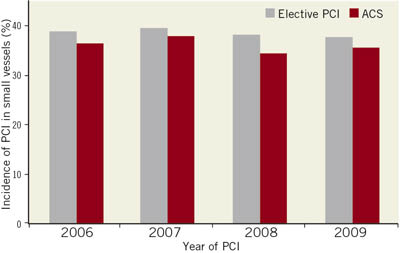
Figure 1. Incidence of PCI in vessels of <2.8 mm in patients with stable coronary artery disease and patients with acute coronary syndromes in the German ALKK-PCI registry.
Today stents are used in over 90% of PCI procedures. With bare metal stents the angiographic restenosis rate in small vessels ranges from 25 to nearly 50%, depending on the stent length and the presence or absence of diabetes (Figure 2). In addition, randomised clinical trials comparing balloon angioplasty and bare metal stents in small vessels failed to show an advantage of bare metal stents. With the introduction of drug-eluting stents, the restenosis rate in small vessels was able to be reduced. In randomised clinical trials, the angiographic angiographic restenosis rate is about 20%, and ischaemic driven target lesion revascularisation is about 10% in small vessels. In registries, the target vessel revascularisation rate in small vessels 6-12 months after DES implantation is higher than 10%.
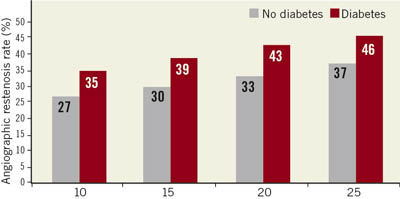
Figure 2. Binary restenosis rate in vessels of 2.5 mm diameter treated with bare metal stents according to the stent length.
In the German CYPHER registry only 21% of DES were implanted in small vessels, because interventionalists do not treat these vessels which have a probability of restenosis with DES. The reasons might include inability to access the vessel with a stent, not enough myocardium supplied by the vessel and cost issues.
Anatomical considerations
Late lumen loss is a well-accepted parameter for the development of restenosis. However, geometrical considerations indicate ahigher impact of late lumen loss on restenosis with smaller vessel size (Figure 3).
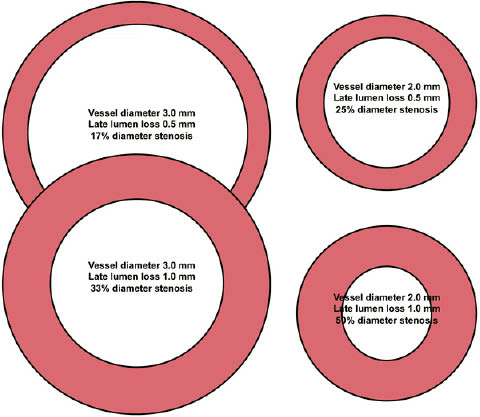
Figure 3. Impact of vessel diameter and late lumen loss on diameter stenosis.
PEPCAD I study
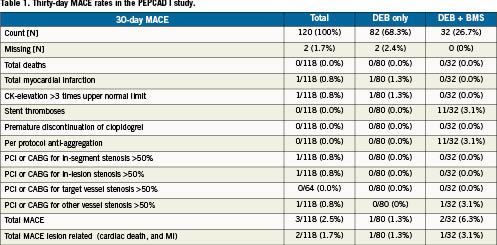
In the PEPCAD-I trial a total of 120 subjects with small vessel disease (vessel diameter >2.2mm and <2.8mm) with stable angina or unstable angina have been enrolled. The objective of this study was to assess the safety and efficacy of the paclitaxel-eluting PTCA balloon catheter (3µg/mm2 balloon surface area) in the treatment of significant (>70% and <100%) stenoses in native coronary arteries with reference diameters from ≥2.25 mm to ≤2.8 mm and <22 mm in length for procedural success and preservation of vessel patency. In four cases lesion crossing was not successful, and in two cases protocol violators occurred. Out of the remaining 114 subjects, 82 have been treated only with the DEB. In the remaining 32 cases the investigators decided for an additional stent implantation due to, e.g., dissections or unfavourable recoil. Thirty-day MACE events are presented in Table1 and the 6-month MACE and quantitative angiographic data are provided in Table2. As outlined in the study synopsis, late lumen loss, binary restenosis rates, TLR were the primary endpoints; whereas MI, death, bleeding complication and thrombotic events were part of the safety assessment. Since TLR is part of the MACE, it may serve as an efficacy market for SeQuent® Please as well. Due to the “single arm” design of this pilot trial, control data are not available.
In the PEPCAD I patient population that was treated with the DEB only, the late lumen loss (LLL) was 0.178±0.375 mm. When combined with a bail out stent, the LLL increased significantly to an overall value of 0.727±0.743mm, which was mostly due to “geographic mismatch”, i.e., the BMS was longer than the DEB or the BMS was implanted outside the “medicated vessel” segment (DEB landing zone).
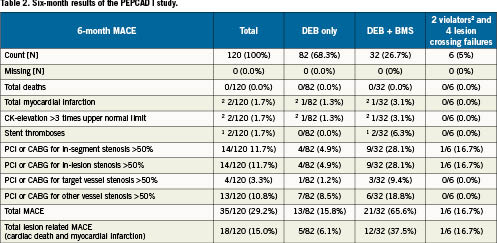
The binary restenosis rate in patients treated with DEB only and DEB + BMS are given in Figure 4.
Figure 4. Six-month binary restenosis rate in PEPCAD-I.
From these results it was concluded that the DEB Sequent® Please for the treatment of small coronary vessels is safe and achieved a high procedural success rate. A DEB only strategy was associated with a low restenosis rate of 5.5%. Additional stenting was necessary in about one-quarter of the patients. In patients with a DEB longer than the BMS with no geographical mismatch, the restenosis rate was about 20%, while there was a very high restenosis rate in patients with geographical mismatch. Therefore the DEB must overlap the stented area to avoid these high restenosis rates.
Treatment recommendations
The results of the PEPCAD I study formed the basis for the treatment recommendations which are published in this special issue of EuroIntervention.
Summary
A “DEB only” strategy might be a reasonable and attractive approach in small vessels of <25 mm lesion length. It avoids further reducing the lumen of the already small vessel with the stent struts. This strategy includes the use of BMS spot stenting in the case of dissections type C or higher (Figure 5). The upcoming DEB-only SVD worldwide registry will evaluate the efficacy and safety of a DEB only strategy according to the treatment recommendations in patients with small vessel disease.
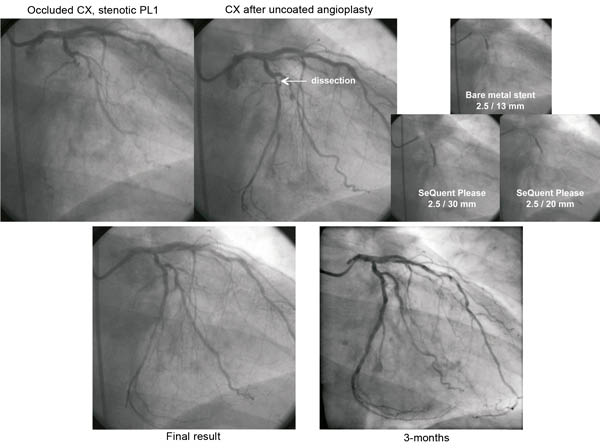
Figure 5. A DEB only strategy in a patient with chronic occlusion of the CX and complex stenosis of the PL1-CX. Recanalisation of CX with conventional PTCA, followed by 13 mm spot bare metal stent due to a dissection, and finally local drug delivery with 30 mm drug-coated balloon. Treatment of the PL1-CX lesion with conventional PTCA followed by 20 mm drug-coated balloon without additional stent implantation.
Conflict of interest statement
Both authors have received research grants and speakers fees from B.Braun, Germany.

