Abstract
Background: Although recent studies have reported that drug-coated balloons (DCB) are non-inferior to drug-eluting stents (DES) for the treatment of native coronary arteries in a specific population, there is no available information concerning vasomotion after treatment with DCB.
Aims: The aim of this study was to prospectively compare coronary vasomotion in patients with small coronary artery disease treated with DCB versus DES.
Methods: Forty-two native lesions (2.0-3.0 mm) treated in our institution were randomly assigned to the DCB arm (n=19) or the bioabsorbable polymer everolimus-eluting stents arm (n=23) after successful predilation. At eight months after treatment, endothelium-dependent and -independent vasomotion was evaluated with intracoronary infusions in incremental doses of acetylcholine (right coronary artery: low dose 5 μg, high dose 50 μg; left coronary artery: low dose 10 μg, high dose 100 μg) and nitroglycerine (200 μg). The mean lumen diameter of the distal segment, beginning 5 mm and ending 15 mm distal to the edge of the treated segment, was quantitatively measured by angiography.
Results: The luminal dimension in the treated segment did not differ between groups at the follow-up angiography. The vasoconstriction after acetylcholine infusion was less pronounced in the DCB arm than in the DES arm (low-dose: 6±13% vs –3±18%, p=0.060; high-dose: –4±17% vs –21±29%, p=0.035). The response to nitroglycerine did not differ between groups (17±13% vs 17±22%, p=0.929).
Conclusions: Vasoconstriction after acetylcholine infusion in the peri-treated region was less pronounced in the DCB arm than in the DES arm, suggesting that endothelial function in treated coronary vessels could be better preserved by DCB than by new-generation DES.
Introduction
Drug-eluting stents (DES), particularly new-generation DES, have been widely used for the treatment of coronary artery disease to reduce both the rate of restenosis and the subsequent need for target lesion reintervention. However, several problems related to stent implantation itself remain unresolved, such as stent thrombosis and stent fracture1. Endothelial dysfunction after stent implantation is another such issue and could lead to serious cardiac events, such as myocardial infarction, fatal arrhythmias, and sudden cardiac death2. Although the exact mechanisms underlying endothelial dysfunction after DES implantation remain unknown, possible causes include delays in endothelial repair due to antiproliferative drugs, the direct effects of such drugs or polymers, and mechanical injury by metallic mesh34.
In recent studies, treatment with drug-coated balloons (DCB) for de novo coronary artery disease has yielded comparable outcomes to treatment with DES in terms of target lesion revascularisation and major adverse cardiac events567. DCB were therefore suggested as an alternative to DES for some lesions. However, despite the effectiveness of DCB for de novo coronary artery disease, the physiological responses, especially endothelial function, after treatment with DCB have not been fully investigated. The purpose of this study was to prospectively compare coronary vasomotion in patients treated with DCB or new-generation DES.
Methods
STUDY DESIGN
This study, “The Degree of endothelial Dysfunction caused by treatment of De novo coronary lesion with Drug-coated balloon as compared to Drug-eluting stent” (the D5 study) was a prospective, open-label, randomised trial comparing vasomotion in native coronary arteries treated with DCB versus new-generation DES. This study was carried out according to the guidelines of the Declaration of Helsinki and was approved by the ethics committee at our institution. All patients in this study provided signed informed consent. The present study was registered at the University Hospital Medical Information Network Clinical Trials Registry in Japan (UMIN000032152).
From April 2018 to March 2020, we enrolled patients suffering from de novo lesions scheduled to undergo percutaneous coronary intervention (PCI) in our institution. The inclusion criteria for patients were age ≥20 years and presentation with stable angina or documented silent ischaemia with de novo coronary lesions. The inclusion criteria for lesions were a reference vessel diameter of 2.0-3.0 mm (in Japan, DCB is allowed only for in-stent restenosis and small native coronary lesions) and a lesion length of ≤25 mm. The exclusion criteria for patients were left ventricular ejection fraction <30%, acute myocardial infarction within the previous 48 hrs, known renal failure (serum creatinine >2 mg/dl), known hypersensitivity to or contraindication for the required medication, history of vasospastic angina, or life expectancy <1 year. The exclusion criteria for lesions were left main lesions, ostial lesions, heavily calcified lesions, thrombotic lesions, or previous stent implantation or another stenotic lesion in the treated vessel. We also excluded patients with restenosis or development of de novo significant stenosis (>70%) at the follow-up angiography.
All study patients received oral aspirin (100 mg/day) and clopidogrel (75 mg/day) for at least five days before PCI. Heparin was administered before and during the procedure to maintain an activated clotting time >250 s. All lesions were predilated using a regular balloon, a scoring balloon or a cutting balloon with a balloon-to-vessel ratio of 0.8-1.0. If an acceptable angiographic result was obtained (i.e., Thrombolysis in Myocardial Infarction [TIMI] 3 flow, residual stenosis <30% and no flow-limiting dissection), patients were randomly assigned in a 1:1 ratio to receive either angioplasty with DCB or implantation of DES according to a computer-generated block randomisation table (random sequences of block sizes between 4 and 8). If a suboptimal result occurred, provisional stenting with DES was performed and the patient was classified into a non-randomised arm. In the DCB arm, the lesion was followed by only one dilation with DCB (SeQuent® Please; B. Braun Melsungen AG, Melsungen, Germany) for ≥30 s. The diameter and length of the DCB were selected with a balloon-to-artery ratio of 1.0 and an overlap of ≥2 mm on each edge of the predilation balloon-treated segment, respectively. In the DES arm, stent implantation was performed as standard practice. Post-dilation was left to the discretion of the operator. All DES in the study were bioabsorbable polymer-coated everolimus-eluting stents (SYNERGY™; Boston Scientific, Marlborough, MA, USA). After the procedure, dual antiplatelet therapy was recommended for ≥3 months in the DCB arm and ≥6 months in both the DES arm and non-randomised arm, followed by aspirin monotherapy indefinitely.
The primary endpoint of this study was endothelial function adjacent to the treated segment by a vasomotion test at the follow-up angiography. Secondary endpoints included angiographic measurements, intravascular ultrasound (IVUS) parameters, and clinical outcomes according to the Academic Research Consortium criteria8.
EVALUATION OF ENDOTHELIAL FUNCTION
All patients were scheduled to undergo clinical and angiographic follow-ups at eight months. Endothelial function was evaluated by an acetylcholine (ACh) vasomotion test at the follow-up angiography9. Any medications with potential effects on vasomotor responses, including calcium channel blockers and long-acting nitrates, were withheld for at least 48 hours before the angiography. Endothelium-dependent vasomotor response was assessed by an intracoronary infusion of incremental doses of ACh (5 μg and 50 μg for right coronary artery, 10 μg and 100 μg for left coronary artery; estimated intracoronary concentrations of 3.4×10−7 and 3.4×10−6 mol/l, respectively) for 1 minute. Subsequently, an intracoronary bolus injection of nitroglycerine (NTG, 200 μg) was administered to assess the endothelium-independent vasomotor response. A 3-minute period was allowed to elapse between each drug infusion. Throughout the procedures, the heart rate, the systemic blood pressure, and the electrocardiogram were continuously monitored.
During the ACh vasomotion test, projection of the coronary angiography (CAG) was fixed as the most suitable to show the artery of interest, without overlapping side branches and with less foreshortening. Offline quantitative coronary angiography (QCA) was performed using coronary angiography analysis software (CAAS version 5.9; Pie Medical Imaging, Maastricht, the Netherlands). Calibration of the system was based on dimensions of the guiding catheter. The mean lumen diameter of the distal segment, beginning 5 mm and ending 15 mm distal to the end of the treated segment, was measured to evaluate vasomotor responses and was the primary endpoint of this study10. The definition of each segment is shown in Figure 1. The same segments were identified by anatomical landmarks and were assessed at the baseline, after each dose of ACh and after the NTG bolus. Changes in the coronary diameter, in response to the ACh and NTG coronary infusions, were expressed as percentage changes in mean lumen diameter versus baseline angiograms. Measurements were performed by two independent, experienced investigators. Intra- and inter-observer variability for QCA were assessed in the same recordings of 10 randomly selected patients by intraclass correlation coefficients (ICC) and showed excellent agreements (consistency ICC 0.973, 95% CI: 0.950-0.986 and ICC 0.952, 95% CI: 0.915-0.975, respectively).
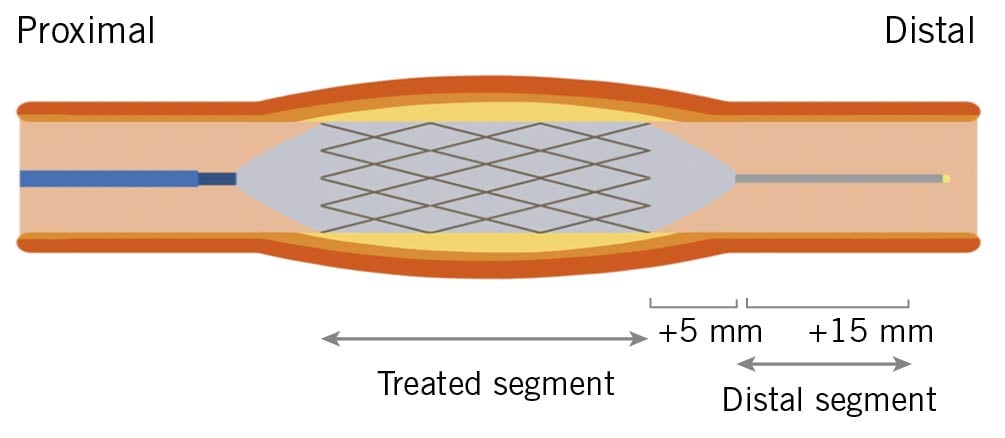
Figure 1. Definition of each segment. A representation of the segment treated with the drug-coated balloon or drug-eluting stent. The distal segment begins 5 mm and ends 15 mm distal to the end of the treated segment.
INTRAVASCULAR ULTRASOUND
Before the pre- and post-procedure image acquisitions, 200 μg of intracoronary NTG was routinely administered. At the eight-month follow-up, an IVUS examination was performed after the ACh vasomotion test. The IVUS imaging was performed using a high-definition 60 MHz IVUS system (AltaView™; Terumo Corp., Tokyo, Japan) with an automatic pullback device at 3 mm/s. Offline analyses were performed using an IVUS imaging system (VISICUBE; Terumo Corp., Tokyo, Japan). The geometric characteristics of the treated segments were analysed, and lesions were confirmed using identical landmarks such as side branches, calcification, pericardium, vein, and plaque shape.
Geometric quantitative IVUS analyses were performed according to the criteria of the clinical expert consensus document11. The leading edge of the lumen and the external elastic membrane were traced using manual planimetry and the plaque area was defined as the area between these leading edges. As cross-sectional images were obtained at 1.0 mm intervals, the total plaque and lumen volume were calculated using the Simpson rule as the mean plaque and lumen area multiplied by the pullback distance in millimetres. IVUS measurements were performed by an independent examiner who was blinded to the clinical characteristics of participants.
STATISTICAL ANALYSIS
Based on the results of previous studies412, we expected that treatment with DCB would achieve a 5±5% reduction in coronary vasospastic response to ACh compared to treatment with DES. The assumption used for power calculations required a sample size of 16 patients per group to provide 80% power (assuming a standard deviation [SD] of 5%) to detect a 5% difference with a 5% type I error rate for two-sided testing. Given a restenosis rate of 5-10% and some dropouts, the total sample size required is 50 (25 in each arm). Continuous variables are expressed as mean±SD for values showing a normal distribution, or as medians and interquartile ranges for values showing anormal distributions. Categorical data are presented as absolute values and/or frequencies. Baseline group characteristics were compared using a t-test for continuous variables showing a normal distribution or Mann-Whitney's U-test for continuous variables showing anormal distributions, and the chi-square or Fisher’s exact test for categorical variables. Changes in vessel diameter in response to drug infusions among groups were compared with an unpaired Student’s t-test, and interactions between groups were tested by two-way repeated measures analysis of variance (ANOVA). Statistical significance was set at the level of p<0.05. All the statistical analyses were carried out using EZR version 1.53 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), and the graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria)13.
Results
POPULATION AND BASELINE CHARACTERISTICS
From April 2018 to March 2020, 46 patients who were eligible based on the inclusion criteria were enrolled in this study. Nineteen patients were assigned to the DCB arm, twenty-three patients to the DES arm, and four patients to the non-randomised arm because of suboptimal results after predilation. A follow-up angiography was not performed for one patient in the DES arm because of worsening renal function. Therefore, a total of 41 patients (DCB arm, 19 patients; DES arm, 22 patients) underwent angiographic follow-ups. All patients, except one patient in the DES arm who showed a new, significant stenosis in a non-treated vessel, subsequently received an ACh vasomotion test (Figure 2). No major adverse cardiac events including death, target lesion revascularisation, and myocardial infarction, occurred during the follow-up.
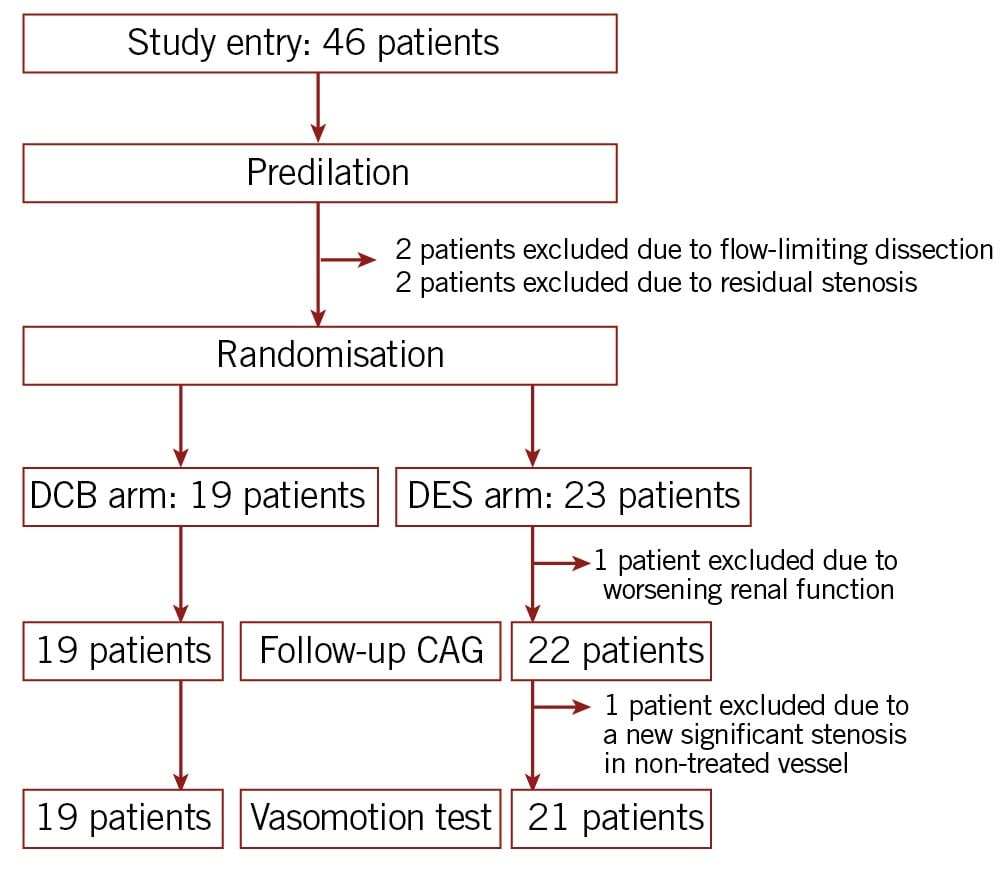
Figure 2. Study flow. CAG: coronary angiography; DCB: drug-coated balloon; DES: drug-eluting stent.
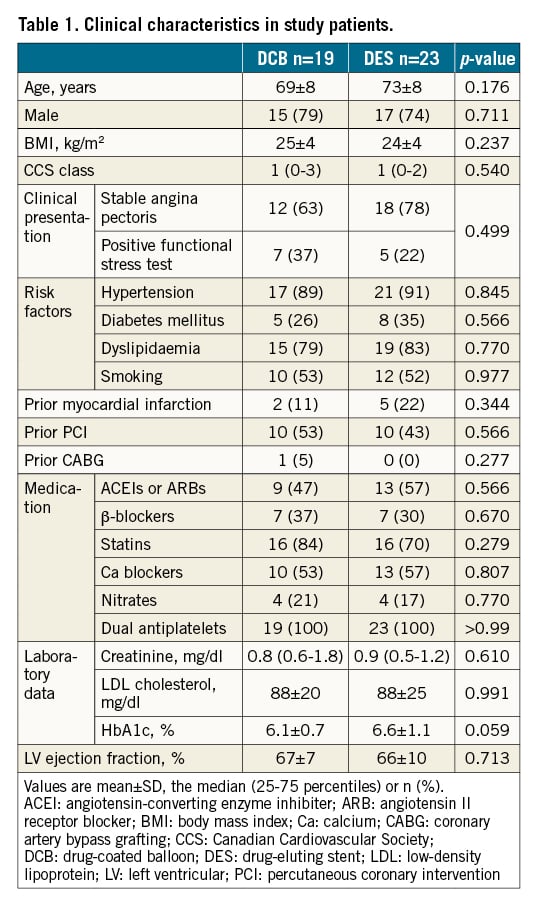
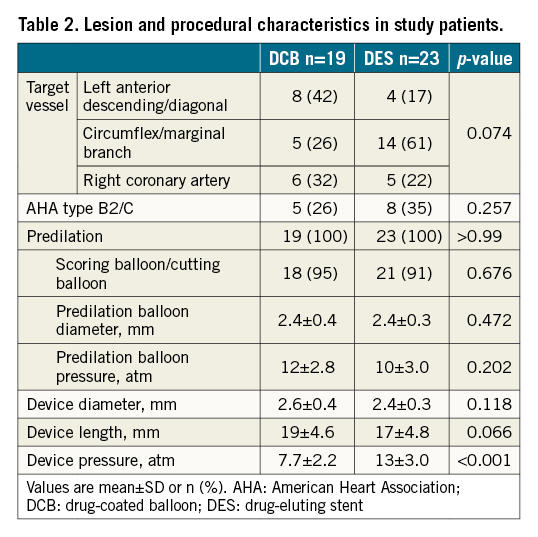
The baseline patient characteristics are summarised in Table 1. No significant differences in the baseline patient characteristics were evident between the groups. Table 2 summarises lesion and procedural characteristics. The patients in the DCB arm were treated with lower pressure than patients in the DES arm due to differences in nominal pressure between each device. No other significant differences in baseline lesion and procedural characteristics were seen between the groups.
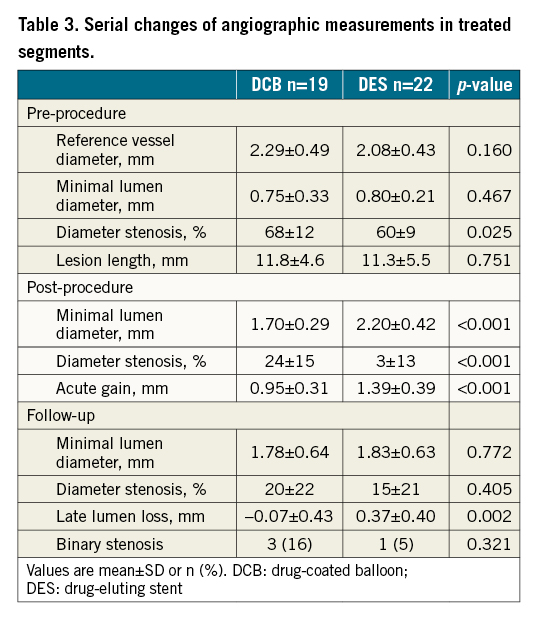
ANGIOGRAPHIC RESULTS FOR THE TREATED SEGMENT
Serial changes in QCA measurements from the treated segments are summarised in Table 3. Measurements showed a slightly higher % diameter stenosis at pre-procedure in the DCB arm compared to the DES arm. After the procedure, lesions treated with DCB showed lower acute gain, therefore lower minimal lumen diameter (MLD) and higher % diameter stenosis than lesions treated with DES. At the follow-up, QCA measurements showed lower late lumen loss in the DCB arm compared to the DES arm. As a result, differences in MLD and % diameter stenosis between groups disappeared.
ENDOTHELIAL FUNCTION ANALYSIS
Representative cases of each arm are shown in Central illustration A. Central illustrations B and C show percentage changes in mean lumen diameter for the evaluation of vasomotion in response to ACh and NTG. In the distal segment (Central illustration B), vasoconstriction after ACh infusion was less pronounced in the DCB arm than in the DES arm (low-dose: 6±13% vs –3±18%, p=0.060; high-dose: –4±17% vs –21±29%, p=0.035), with between-group differences of 9% (95% CI: 0 to 20) after low-dose ACh infusion and 17% (95% CI: 1 to 32) after high-dose ACh infusion. The response to NTG did not differ between groups (17±13% vs 17±22%, p=0.929). In addition, significant interaction of the two groups in the vasomotion test was identified (p=0.037, ANOVA). In contrast, no significant difference between groups was seen in vasoconstriction of the treated segment (Central illustration C, p=0.227, ANOVA). Serial changes in lumen diameter in individual cases are shown in Supplementary Figure 1.
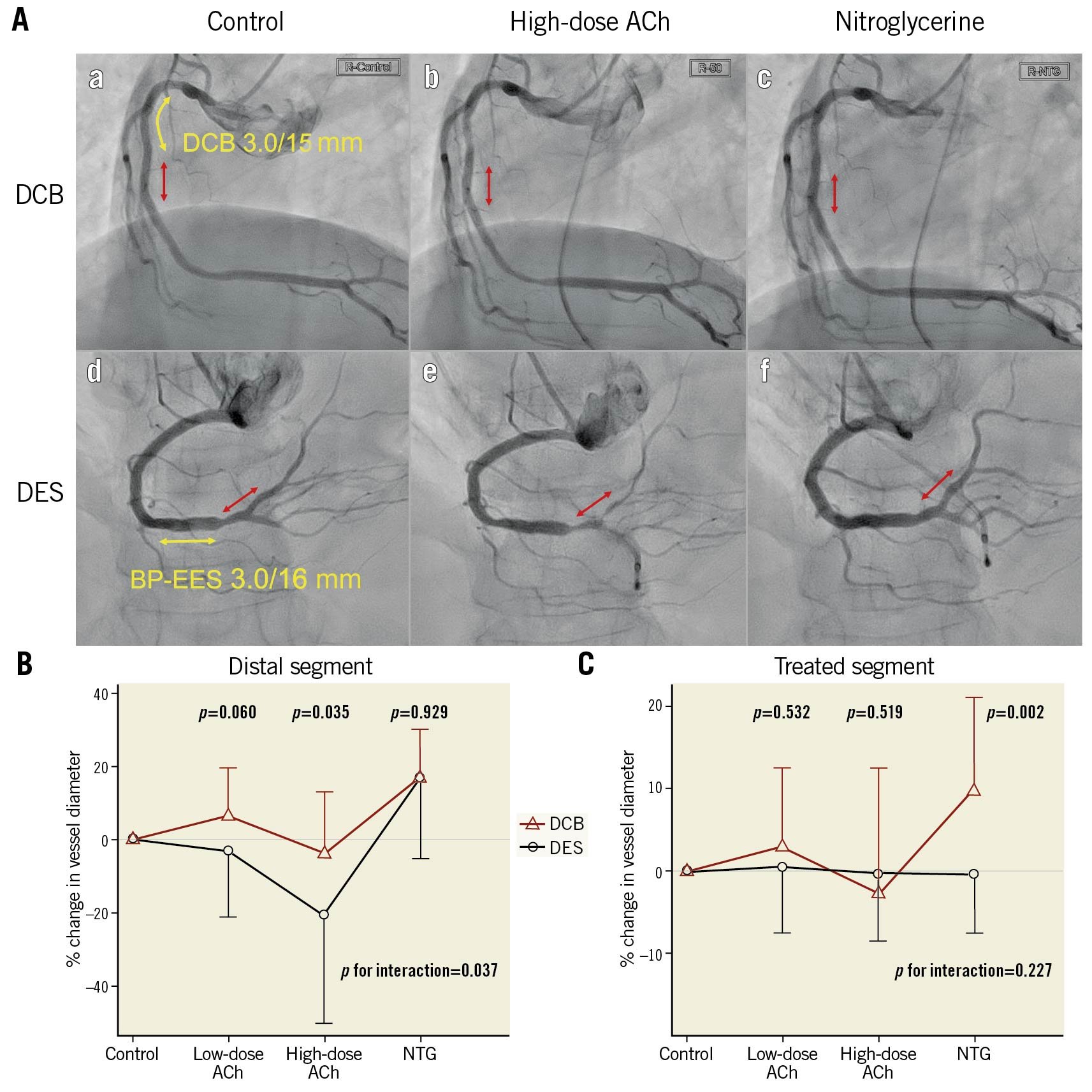
Central illustration. Comparison of vasomotion after DCB versus DES. A. Representative cases of the vasomotion test eight months after treatment with drug-coated balloons (DCB) and drug-eluting stents (DES). Aa and Ad show control coronary angiograms. Ab and Ae show angiograms after infusion of high-dose acetylcholine (ACh). Ac and Af show angiograms after infusion of nitroglycerine (NTG). The red arrows indicate distal segments, as the regions of interest in vasomotion studies. The yellow arrows indicate treated segments. Vasospasm occurred in the distal segment in a patient with DES, but not with DCB. B, C. Percentage change in vessel diameter in response to ACh and NTG in the distal segment and treated segment. Mean values with standard deviation as error bar are shown. P values for interaction indicate differences between the randomised groups and serial drug infusions. BP-EES: bioabsorbable polymer-coated everolimus-eluting stent
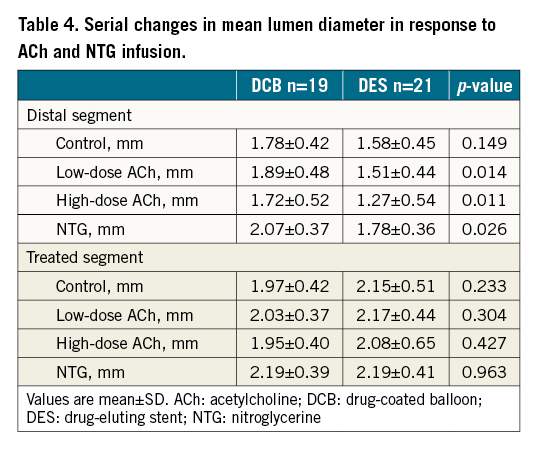
Absolute values of the mean lumen diameter in response to ACh and NTG showed consistent results (Table 4). In the distal segment, the mean lumen diameter in response to ACh was significantly greater in the DCB arm than in the DES arm. On the other hand, in the treated segment, no differences between groups were seen in the lumen size in response to ACh and NTG. Scatter plots comparing the control mean lumen diameter and the mean lumen diameter following a high-dose ACh infusion in the distal segment are shown in Supplementary Figure 2.
IVUS RESULT FOR TREATED SEGMENT
Procedural and follow-up IVUS measurements are summarised in Supplementary Table 1. IVUS images were analysable for 34 patients (DCB, n=17/19 [89%]; DES, n=17/22 [77%]). Measurements showed a larger plaque area and minimal lumen area in the DCB arm than in the DES arm. No significant differences were found in the mean lumen area at the baseline. Lesions treated in the DCB arm displayed lower acute gain after the procedure, but lower late lumen area loss at the follow-up than the DES arm. These results were consistent with the angiographic results.
Discussion
The current study demonstrated that endothelial vasomotor reactivities after treatment with DCB were better preserved than with DES. In terms of angiographic results in the treated segment, treatment with DCB for de novo coronary lesions could be comparable to treatment with DES at the follow-up period, consistent with previous reports567. To the best of our knowledge, this is the first trial to investigate endothelial vasoreactivity after DCB treatment, finding better preservation of endothelial vasomotor activity after treatment with DCB compared to new-generation DES.
In patients with coronary artery disease, DES dramatically reduces both the rate of restenosis and the subsequent need for target lesion reintervention. However, many studies have reported endothelial dysfunction after stent implantation. Endothelial dysfunction was first reported after bare metal stent implantation4, and multiple trials have since demonstrated severe endothelial dysfunction after first-generation DES implantation1415. More recent studies have shown that new-generation DES also induced endothelial dysfunction, although to a lesser degree than first-generation DES91012. On the other hand, an in vitro study using porcine coronary arteries showed that endothelium-related vasodilation was better preserved in vessels treated with DCB than in vessels treated with DES implantation16. However, coronary endothelial function after treatment with DCB in clinical practice has not been fully explored.
Although the pathophysiology of the endothelial dysfunction remains unknown, several possible causes have been suggested so far, including delayed endothelial repair, direct effects of antiproliferative drugs or polymers, and mechanical injury by metallic mesh3417.
All DES used in the present study were SYNERGY™. This bioabsorbable, polymer-coated, thin-strut DES has shown excellent endothelial stent coverage in previous in vitro and in vivo studies181920. However, the function and maturation of newly developed endothelial cells after new-generation DES implantation have not been well studied. A study using optical frequency domain imaging reported dissimilar properties of the neointima after implantation of a bioresorbable scaffold and DES21. The stent might be covered with new endothelium when treated with DES, but the original endothelium remains buried under the stent. On the other hand, when treated with DCB, original endothelial cells may remain in the treated vessels because of the absence of a metallic barrier. The functional difference between the newly developed and original endothelium might lead to the results shown in the present study.
In this study, the direct influence of antiproliferative drugs and polymers might be negligible. After the release of the paclitaxel from SeQuent® Please and its adsorption by the vessel wall, the concentration of paclitaxel in the vessel wall declines to almost zero within 24 hours22. On the other hand, after implantation of the SYNERGY™ stents, everolimus is released for 90 days. In terms of polymers, SYNERGY™ stents also have an abluminal poly-lactide-co-glycolide polymer that is resorbed within four months despite of the ensured coating integrity232425. Therefore, at follow-up angiography eight months after the initial procedure, the remaining antiproliferative drugs and polymer should exert minimal influence on endothelial function.
Mechanical injury by metallic mesh could affect the vasomotion of adjacent arterial segments. A previous study reported that more severe endothelial dysfunction was observed in the long-term after stenting as compared to plain ballooning or directional atherectomy4. Another study showed that the strong, permanent passive stretching of the endothelial and muscular layers by stenting elicited hypersensitivity of the adjacent arterial segments to endothelin, probably through endothelin-related contraction due to interactions with G protein-coupled receptors, or the production of asymmetric dimethylarginine increasing vascular resistance16. Such observations could provide one explanation for the impairment of endothelial vasomotor reactivity in the DES arm.
Several studies have reported that DCB for de novo coronary lesions was associated with non-inferior clinical outcomes, even when compared to new-generation DES567. In terms of angiographic results, treatment with DCB tended to be associated with smaller lumen diameter and acute gain, but also with lower late lumen loss, and non-inferior results to DES were achieved at the angiographic follow-up526. On this point, the present results were consistent with findings from those studies. More interestingly, according to a recent meta-analysis of DCB for de novo coronary lesions, the incidence of all-cause mortality and myocardial infarction was lower with DCB treatment, despite the similar or slightly worse angiographic results27. This paradoxical finding may derive from the difference between the presence and absence of permanent implants. The vasoconstriction provoked after stent implantation could result in blood flow reduction and exacerbation of nonlaminar flow within the stented vessel. These likely link to increases in inflammatory and thrombotic risks and lead to stent-related adverse events22829. On the other hand, DCB for de novo coronary lesions can better preserve the physiological functioning of vessels. Therefore, a stentless strategy with DCB may be a safer treatment for a particular subset of de novo coronary lesions.
Limitations
First, the baseline endothelial function was not investigated. The initial endothelial function test could not be applied, because all study patients had significant coronary lesions and the safety of the ACh vasomotion test was a concern. In addition, several lesion characteristics, especially in IVUS examination, were different between groups. It is reported that epicardial spasm is more often located in the distal small coronary segments. Therefore, there was a concern regarding the influence of vessel size on vasoconstriction. However, in this present study, there were no significant correlations between percentage changes in mean lumen diameter to high-dose ACh in the distal segment and vessel size, such as the mean diameter of the distal segment in QCA (r2=0.274, p=0.087) and the mean vessel area of the treated segment measured with IVUS (r2=0.179, p=0.297). Second, after randomisation, one patient assigned to the DES arm was excluded before angiography and another patient could not receive the vasomotion test. However, the baseline lesion and procedural characteristics were still well balanced among the two groups in the analysis of patients who could receive angiography or vasomotion tests. In addition, similar tendencies could be seen in serial changes in QCA measurements of treated lesions when we limited the analysis to patients who could receive vasomotion tests. Third, the present study investigated only patients with small coronary artery diseases. Therefore, the applicability of the current findings may be limited in larger (≥3.0 mm reference diameter) coronary artery diseases. Fourth, racial differences might have influenced the present results. Asian populations exhibit a higher degree of coronary vasoconstriction in response to ACh infusion than Caucasians30. Fifth, the rate of scoring/cutting balloon use is remarkably high. In Japan, the scoring/cutting balloon for lesion preparation is popular. However, this may not reflect the practice outside of Japan. Finally, the present study was a single-centre study with a small sample size, so the results should be considered preliminary. In addition, the real-world clinical implications of the differences in endothelial function between each device remain unclear. A larger number of subjects and a longer follow-up of clinical outcomes are crucial to confirm the efficacy of DCB.
Conclusions
This study provides evidence that treatment with DCB for de novo coronary lesions could be comparable to treatment with DES in terms of the angiographic results, and showed that vasoconstriction after ACh infusion in the peri-treated region was less pronounced in lesions treated with DCB than in those treated with DES. This suggests that the endothelial function of coronary vessels treated with DCB may be better preserved than that of vessels treated with new-generation DES.
Impact on daily practice
Endothelial dysfunction after PCI could lead to serious cardiac events, such as myocardial infarction, fatal arrhythmia, or sudden cardiac death. The present study is the first to reveal that endothelial function is better preserved in coronary vessels treated with DCB than with new-generation DES. The present results suggest that DCB for de novo coronary lesions can preserve the physiological functioning of vessels better than new-generation DES, which may be a beneficial effect of the "leave nothing behind" concept of DCB.
Acknowledgements
The authors thank Daisuke Sakamoto from Osaka General Medical Center (Osaka, Japan) for his technical assistance, and Kanako Ueda, Yumiko Sugie, and Junko Tsugawa from Osaka General Medical Center (Osaka, Japan) for their secretarial assistance.
Funding
This study was partially funded by Osaka Heart Club (Osaka, Japan), which has no relationship with the industry. The funder supported the conduct of the study.
Conflict of interest statement
Y. Sotomi received research grants and speaker honoraria from Abbott Vascular Japan, Boston Scientific Japan, Terumo, Japan Lifeline, Biosensors, Medtronic, Daiichi-Sankyo, Bayer, Boehringer Ingelheim, and Bristol-Myers Squibb and is an endowed chair funded by Terumo, Asahi Intecc, Nipro, and Shimadzu Corporation. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

