- balloon angioplasty
- drug-coated balloon
- drug-eluting stent
- late loss
- restenosis
- small coronary vessels
Abstract
Lesions in small coronary vessels comprise a challenging disease subset in contemporary interventional practice. Due to the limitations of mechanical methods for preventing restenosis in small vessels, such lesions have been considered the Achilles’ heel of bare metal stenting, with little marginal antirestenotic efficacy in comparison with balloon angioplasty. In contrast, modalities employing biological or pharmaceutical methods for restenosis prevention – in particular drug-eluting stents (DES) – have demonstrated greatest antirestenotic advantage in vessels with reference diameter under 2.8 mm. Moreover, lesions in small vessels served as in important stress test in uncovering efficacy differences between comparator DES platforms. Drug-coated balloon therapy has shown encouraging results in certain subgroups – most notably in restenosis within bare metal stents. However, what limited data exists to date does not suggest a clear role for this modality in lesions in small coronary vessels. On the basis of sound scientific principle and accumulated trial data, DES therapy represents the treatment of choice for this condition, simultaneously combining high acute gain with low late loss. While concerns related to late adverse events after DES implantation focus attention on the need for interventional modalities delivering high antirestenotic efficacy with a minimum of vascular wall toxicity, it may well transpire that in this disease subset novel stent platforms – such as fully bioabsorbable DES – represent a more promising way forward than drug-coated balloons.
Introduction
The principal cause of treatment failure following percutaneous coronary intervention is restenosis of the intervened segment.1 As such, the advent of drug-eluting stent (DES) technology represented a very significant forward step and facilitated the expansion of percutaneous intervention to a number of challenging subsets –including small vessels, bifurcation disease and in-stent restenosis– that were formerly limited by unacceptable rates of restenosis.2,3 However, the undoubted efficacy of DES in preventing restenosis has been plagued somewhat by concerns regarding delayed healing of the stented arterial segment.4 This pathophysiological process seems to underlie a spectrum of late adverse events following DES therapy, including stent thrombosis, marginal erosion of luminal calibre (“late luminal creep”) and impaired vascular responsiveness.5-7 Although such delayed healing is undoubtedly multifactorial in aetiology, persistent inflammatory response to non-erodible polymer coatings seems to play a central role.8 Concerns related to delayed healing have served as an impetus for the development of newer technologies which deliver high antirestenotic efficacy with lesser impact on arterial healing. Of these newer therapies drug-coated balloon (DCB) technology has shown particular promise in a number of lesion subtypes.9
Small vessel disease – the Achilles’ heel of bare metal stenting
Obstructive disease of small coronary vessels remains a particularly challenging issue in routine clinical practice. It is well documented that vessel calibre correlates inversely with antirestenotic efficacy after percutaneous intervention.10,11 This is because the magnitude of late loss is typically independent from the reference vessel size. Consequently, smaller calibre vessels are less able to accommodate a given degree of late loss –whether due to mechanical processes like vessel recoil and constrictive remodelling (predominantly seen in angioplasty patients), or tissue ingrowth caused by neointimal hyperplasia (in stent-treated patients)– without exceeding the threshold where repeat revascularisation becomes necessary. Indeed in one analysis of 3,370 bare metal stent treated patients, we found that small vessel size was the strongest of all clinical, lesion and procedural risk factors for restenosis.12
At the same time, although the introduction of bare metal stent therapy undoubtedly represented a key milestone in percutaneous intervention, we should remember that in the specific subset of patients with small vessel disease bare metal stent therapy has encountered its greatest limitations.13 As an example, in the Intracoronary Stenting or Angioplasty for Restenosis Reduction in Small Arteries (ISAR-SMART) trial, in which 404 patients with lesions in vessels of calibre between 2.0 mm and 2.8 mm were randomised to balloon angioplasty versus stenting with MULTILINK bare metal stent, there were no significant differences between the two treatment groups in terms of angiographic or clinical restenosis at follow-up. (Figure1A)14 The lesson from this study might be framed as follows: the absolute magnitude of extra acute gain achievable by stenting over balloon angioplasty in small vessel disease is relatively small and does not compensate for the reduced capacity of small calibre vessels to accommodate for increased neointimal hyperplasia seen after stent implantation. Or put another way, small vessels limit the degree that we can attenuate restenosis by purely mechanical means. The corollary of this finding is that apriori small vessels represent an ideal testing ground for specific biological approaches to restenosis prevention. Of such local pharmacological approaches DES therapy has proved the most effective method to date.3
DES versus bare metal stents
Drug-eluting stents have proven to be very effective at preventing restenosis after percutaneous intervention by targeting the biological mechanisms that underlie neointimal hyperplasia and by providing an effective delivery system that result in adequate local tissue drug concentrations.
In the setting of randomised controlled trials, DES have shown consistent reduction in requirement for repeat revascularisation –with a relative risk of the order of 0.30-0.50– when compared with bare metal stents.6,15-17 However, on closer inspection it can be observed that the bulk of this reduction in restenosis is driven by outcomes in patients with small vessels.
For example, in a 500 patient randomised trial we found that overall patients treated with Cypher® DES (Cordis, Johnson & Johnson, Warren, NJ, USA) as opposed to bare metal stents had lower rates of angiographic restenosis (relative risk, 0.33, p<0.001) and repeat revascularisation (relative risk 0.38, p<0.001).18 Subgroup analysis was then performed for patients with reference vessel diameter above and below 2.8mm. For smaller vessels, angiographic restenosis rates were 7.0% with DES versus 34.2% with bare metal stent (p<0.001). Against this for larger vessels, angiographic restenosis rates were not different between stent types (10.0% with DES versus 13.1% with bare metal stent; p=0.52). Asimilar effectiveness concordance was reported in a vessel-size subgroup analysis of the Basel Stent Kosten-Effektivitäts Trial (BASKET).19 On the other hand the recently published Basel Stent Kosten-Effektivitäts Trial–Prospective Validation Examination (BASKET-PROVE) trial examined outcomes in patients with treated lesions in reference vessels >3.0mm and found no statistically significant difference between bare metal stents versus Cypher DES or Xience™ DES (Abbott Laboratories. Abbott Park, Il, USA).20
Looking at randomised trial data specifically addressing the issue of lesions in small vessels, the Sirolimus-Eluting Stent in the Prevention of Restenosis in Small Coronary Arteries (SES-SMART) study compared outcomes following treatment with Cypher DES versus bare metal stenting in patients with lesions in reference vessels ≤2.75mm.21 At 8-month follow-up angiography binary in-segment restenosis rate was 10% in patients receiving asirolimus-eluting stent against 53% of those treated with bare metal stenting. (Figure 1B) The magnitude of relative risk is striking at 0.18 (95% confidence interval 0.10-0.32; p<0.001) and confirms the impression that the greatest antirestenotic advantage with DES is found in vessels with small reference diameter.
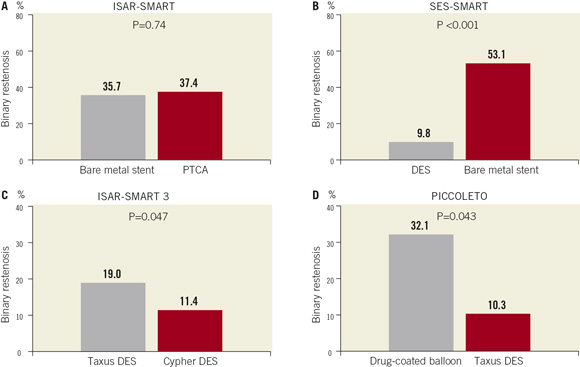
Figure 1. Comparative efficacy studies in lesions in small coronary vessels.Rates of in-segment binary angiographic restenosis at surveillance angiography in randomised trials of (A) balloon angioplasty versus bare metal stenting (ISAR-SMART); (B) bare metal stenting versus drug-eluting stenting (SES-SMART); (C) drug-eluting stents (Cypher versus Taxus) (ISAR-SMART 3); and (D) drug-coated balloon versus drug-eluting stent (PICCOLETO)
Lesions in small vessels – the stress test of DES performance
Although it was not always the case, it is now well accepted that not all DES are equal and that significant differences exist between approved DES platforms with respect to their effectiveness in inhibiting restenosis. Such differential performance efficacy was first manifest in high-risk patient and lesion subsets – including patients with diabetes mellitus, intervention for in-stent restenosis and stenting in small coronary vessels.22-24
In the non-inferiority Intracoronary Stenting or Angioplasty for Restenosis Reduction in Small Arteries 3 (ISAR SMART 3) trial a total of 360 patients undergoing intervention in native coronary vessels with a diameter of ≤2.80mm received randomly Taxus® (Boston Scientific, Natick, MA. USA) or Cypher DES. Regarding the primary endpoint of in-stent late luminal loss, the Taxus stent failed to meet non-inferiority criteria (late loss was 0.32mm higher with Taxus (upper 95% boundary 0.42mm; pnon-inferiority=0.99). In keeping with this, binary restenosis was significantly higher with Taxus (19.0% versus 11.4%, psuperiority=0.047) (Figure1C). These findings highlight that the importance of relative antirestenotic efficacy is amplified in the testing setting of small vessel intervention.
The promise of drug-coated balloon therapy: is it specific to restenotic lesions?
In the last few years, DCB therapy has emerged as promising therapeutic intervention for the management of obstructive cardiovascular disease.9 The principle of this novel technology is that effective prevention of restenosis may be achieved by the short-term transfer of antiproliferative drug to local arterial tissue by means of single balloon angioplasty dilatation typically lasting 30-60 seconds. Its main attraction is that there is no foreign body implanted, and consequently the risk of late inflammatory response to device components (such as the polymer coatings of DES platforms) is obviated.
Initial published data with drug-coated balloon catheters was encouraging. The Treatment of In-Stent Restenosis by Paclitaxel-Coated Balloon Catheters (PACCOCATH ISR) trial enrolled patients with bare metal stent restenosis and randomised participants to either treatment with a balloon catheter coated with paclitaxel at a concentration of 3µg/mm2 (PACCOCATH, Bayer Schering Pharma, Berlin, Germany) versus angioplasty with an uncoated balloon catheter.2 At 6-months, the primary endpoint of in-segment late luminal loss was 0.09±0.49mm with the DCB versus 0.76±0.86mm with the uncoated balloon catheter (p=0.003).
Similarly promising results were observed in the Paclitaxel-Eluting PTCA-Catheter In Coronary Disease (PEPCAD-II) randomised trial where patients with in-stent restenosis were treated with either the Sequent Please paclitaxel-coated balloon catheter (B.Braun, Melsungen, Germany) as compared with repeat stenting with the Taxus DES.26 At 6-9 months, the primary endpoint of late luminal loss was significantly lower with the DCB compared with the DES (0.17±0.42mm vs. 0.38±0.61mm, p=0.03) though this finding must be considered with considerable caution as, for reasons discussed in more detail later, the choice of primary endpoint is open to question. In terms of 12-month clinical results, these also tended to favour the DCB.
However, it should be acknowledged that in both these cases, positive results were obtained in patients presenting with restenosis within bare metal stents. This represents a clinical niche perhaps intuitively suited to DCB therapy. The existing rigid stent scaffold opposes the principle mechanical forces contributing to restenosis –namely elastic vessel recoil and constrictive remodelling (though not necessarily acute plaque prolapse). As a result, fewer patients require implantation of a second stent layer, a scenario which we know should be avoided where possible.27
Whether the positive results with DCB therapy in bare metal stent restenosis can be replicated in patients with DES restenosis remains to be seen. It is hoped that data from the ongoing Intracoronary Stenting and Angiographic Results: Drug Eluting Stent In-Stent Restenosis: 3 Treatment Approaches (ISAR-DESIRE-3) trial (clinicaltrials.org identifier NCT00987324) –in which patients with limus-agent DES-restenosis are randomised to plain balloon angioplasty, paclitaxel-eluting stent (Taxus DES) or paclitaxel-coated balloon– will shed some further light on the management of patients with this challenging clinical condition.
The principal concern with DCB therapy is that effective drug delivery and transfer to the target lesion is inherently more challenging with this modality than with therapy based on a fixed implanted device. Indeed the precise mechanism of action of DCB therapy remains somewhat unclear. Preclinical experiments suggest that ≈ 85-90% of drug loaded on a balloon catheter is lost during transit to the diseased lesion.28 Furthermore, when we consider the extensive body of animal model and clinical data available on DES technology, one lesson that we have learned is that controlled drug-release over the first 30 days and in particular over the first 10-15 days is vital for the effective inhibition of neointimal growth over the short- to medium- term.29 Although, it is possible that DCB has some specific advantages over DES therapy in terms of enhanced loading efficiencies on a balloon compared with a stent (greater drug per mm2) and that injury response is less marked when stent implantation is avoided, it is difficult to fully reconcile the problems encountered in fine-tuning the release-kinetics of DES platforms with the apparent efficacy of DCB devices characterised by more rapid drug-release profiles.
Studies of drug-coated balloon therapy in small vessels disease
There are two specific studies published in the literature that have assessed the role of DCB in small vessel coronary disease.
PEPCAD-I
The Paclitaxel-Eluting PTCA-Catheter In Coronary Disease (PEPCAD-I) study was a single-arm non-randomised trial evaluating the safety and efficacy of the Sequent Please balloon catheter which is coated with a mixture of the active drug – paclitaxel at a concentration of 3µg/mm2 –and the X-ray contrast agent iopromide– an excipient to enhance lipophilicity and increase local tissue drug transfer.30 The principal inclusion criteria were lesion length ≤22mm and reference vessel diameter of 2.25-2.8mm. Of 118 study patients, 70% underwent angioplasty with DCB alone; the remaining 30% had suboptimal post-angioplasty results and proceeded to additional bare metal stent implantation. The primary endpoint of mean in-segment late lumen loss was 0.28±0.53mm with binary restenosis occurring in 18% of patients. Overall clinical outcomes at 12 months were also encouraging –the composite of death, myocardial infarction, target lesion revascularisation, and stent thrombosis occurred in 14.4% patients, almost all of which was driven by revascularisation procedures.
Interestingly, the PEPCAD-I investigators also compared outcomes according to treatment received. What they observed is that patients treated with DCB plus additional bare metal stent implantation had significantly poorer outcomes than those treated with DCB alone: late lumen loss was 0.62±0.73 mm versus 0.16±0.38mm (p<0.001) and binary restenosis rate was 45% versus 6% (p<0.001) respectively. Results in this important subgroup are disappointing. Indeed the late loss magnitude observed is more in keeping with that seen with low efficacy DES devices.
The conclusion from PEPCAD-I is that drug-coated balloon therapy in small vessels seemed promising, though in patients with suboptimal angioplasty results, who required additional stent placement, the results were rather less impressive. Moreover the single-arm design permits no insight into how this therapy performs relative to standard clinical practice with DES therapy. In this respect, the recently published PICCOLETO trial provides some additional relevant information.
PICCOLETO
The PICCOLETO study enrolled patients with index lesions in vessels with reference diameter ≤2.75mm.31 Patients were randomised to treatment with the Dior balloon catheter (Eurocor, Bonn, Germany) –coated with paclitaxel microcrystals at a concentration of 3µg/mm2– or Taxus paclitaxel-eluting stent. Groups were well balanced in terms of baseline characteristics. The trial was powered to demonstrate non-inferiority of the drug-coated balloon with respect to in-segment percentage diameter stenosis at 6-month follow-up angiography and planned to enrol 80 patients but was stopped prematurely based on evident superiority in the DES arm. At follow-up percentage diameter stenosis was 43.6±27.4 with DCB versus 24.3±25.1 with DES (psuperiority=0.029) and binary restenosis occurred in nine patients (32.1%) treated with DCB versus three patients (10.3%) treated with DES (p=0.043) (Figure 1D).
The study message from PICCOLETO is that in patients undergoing percutaneous intervention for small vessel coronary disease, treatment with a paclitaxel-eluting catheter was inferior to the current standard bearer therapy of DES implantation. However, it should be acknowledged that the Dior balloon used in PICCOLETO may have lower efficacy than the iopromide-based Braun balloon for example, and it may well be that the same study performed with the latter catheter would have achieved better results.
Limitations inherent in comparison of angioplasty versus stent implantation
Any discussion of the relative merits of a balloon-based versus a stent-based interventional strategy is subject to some important caveats:
1. Firstly, and perhaps most importantly, over and above comparative antirestenotic efficacy, stents provide additional safety benefit over angioplasty in sealing intimal fissures and dissection planes and reducing abrupt vessel closure. As such randomised trials comparing angioplasty versus stent implantation will always produce a significant proportion of patients who cross over from angioplasty to stent implantation by virtue of post-angioplasty perceived high risk of abrupt vessel closure, e.g., residual dissection. This cross-over rate shows considerable variation in the available literature – in the range of 10% and 40%. As an example in the ISAR-SMART study, the rate was 16.5%.14
2. The availability of stent therapy has a knock-on benefit for the antirestenotic efficacy of angioplasty by virtue of providing a safety net that facilitates more aggressive angioplasty dilatation and lower residual stenosis. Indeed it can be nicely demonstrated that rates of restenosis after balloon angioplasty are inversely proportional to the rate of cross-over to stent implantation (Figure 2) –i.e., attention to optimising angioplasty procedural results translates into lower rates of restenosis but at the cost of a higher cross-over to stent implantation.
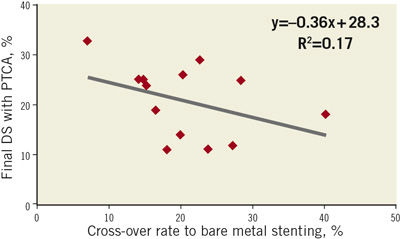
Figure 2. Good results with balloon angioplasty are achieved at the cost of a high cross-over rate to bare metal stenting. Graphic representation of inverse correlation between final percentage diameter stenosis (DS) after angioplasty (PTCA) and rate of cross-over to stent implantation. Figure devised based on meta-analysis data of bare metal stenting versus angioplasty in small vessel coronary disease by Agostini et al.32
3. Caution must be exercised in choice of comparative endpoints when angiographic surveillance data is considered. More specifically it is a tenet of percutaneous intervention that therapies resulting in greater acute gain are subject to relatively higher late loss –“the more you gain, the more you lose” law. For this reason as well as others, late luminal loss is not a suitable comparative endpoint, though net gain and percentage diameter stenosis are.
Take home messages
Lesions in small coronary vessels comprise a challenging disease subset in contemporary interventional practice. Indeed this lesion subset may be considered as both the Achilles’ heel of bare metal stenting as well as an important stress test of modalities with higher antirestenotic efficacy such as DES.
Although drug-coated balloon therapy has shown encouraging results in certain disease subtypes, what limited data exists regarding lesions in small vessels does not suggest a clear role for this modality in de novo coronary disease at present. It cannot be discounted, however, that better performing balloon catheters (with enhanced coating processes) may yet deliver improved outcomes with this technology.
At the current time, and on the basis of sound scientific principle, DES therapy represents the most appropriate treatment modality for stenosis in small vessels, simultaneously combining high acute gain with low late loss. In fact small vessels are the very disease subset where the antirestenotic benefit of DES is most definitively demonstrated.
Finally, while concerns related to late adverse events after DES implantation undoubtedly focus attention on the need for interventional modalities delivering high antirestenotic efficacy with a minimum of vascular wall toxicity, it may well transpire that novel stent platforms –such as fully bioabsorbable DES– represent a more promising way forward than drug-coated balloons.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References

