
Abstract
Aims: We assessed long-term outcomes in patients with extra-small (XS) (≤2.25 mm) and small vessels (SV) (>2.25-2.75 mm) treated with the Resolute zotarolimus-eluting stent (R-ZES).
Methods and results: Data from eight studies including patients with XS or SV were pooled for this analysis. Among 2,141 patients (837 XS, 1,304 SV), three-year cumulative major adverse cardiac events (15.4% vs. 11.5%; adj. HR [95% CI]: 1.3 [1.0, 1.7], p=0.12), target lesion failure (12.4% vs. 9.3%, adj. HR: 1.1 [0.8, 1.5], p=0.56), and target lesion revascularisation (TLR: 6.9% vs. 4.5%, adj. HR 1.4 [0.9, 2.1], p=0.17) were greater in the XS cohort but were not significantly different after propensity adjustment. Target vessel revascularisation occurred more frequently in XS patients in both unadjusted and adjusted analyses (11.2% vs. 7.6%, adj. HR: 1.5 [1.1, 2.1], p=0.02). Stent thrombosis was low in both cohorts (1.2% vs. 0.6%, p=0.09). In the XS cohort, insulin-dependent diabetics had over twofold higher rates of TLR than non-diabetics (13.6% vs. 6.0%, p=0.02).
Conclusions: Long-term lesion-specific results among patients with XS vessels treated with the R-ZES were not significantly different from those among patients with SV, but specific patients with XS vessels (e.g., insulin-dependent diabetics) may remain at high risk for TLR.
Introduction
Percutaneous coronary intervention (PCI) of small vessels (SV) has historically been associated with high rates of acute vessel closure, restenosis, target lesion revascularisation (TLR), and stent thrombosis, particularly in diabetic patients1,2. While first-generation drug-eluting stents (DES) reduced adverse events compared with bare metal stents (BMS), newer-generation DES have improved outcomes further, even among diabetic patients3-6. However, although newer-generation DES suppress late loss and reduce clinical restenosis to the greatest extent seen in modern-day PCI, the performance of these devices within the smallest coronary vessels (with less “tolerated” late loss) is incompletely understood.
The Resolute™ zotarolimus-eluting stent (R-ZES) (Medtronic, Minneapolis, MN, USA) is a second-generation DES that uses a similar cobalt alloy platform and antiproliferative drug to its predecessor, the Endeavor® zotarolimus-eluting stent (E-ZES) (Medtronic), but a different polymer to enhance drug-release kinetics7. Indirect comparisons between R-ZES and E-ZES have demonstrated improved clinical outcomes with the new polymer, particularly a reduction in ischaemia-driven target vessel revascularisation (TVR)8.
An analysis of five R-ZES trials revealed similar outcomes over a two-year follow-up period in patients with small (<2.5 mm) as compared with large (>2.5 mm) reference vessel diameter (RVD)9. The success of R-ZES in SV raises the possibility that even smaller vessels –“extra-small vessels” (XS) ≤2.25 mm in diameter– represent equivalent targets for the improved stent design. We therefore compared outcomes of R-ZES in XS vs. SV (>2.25 to ≤2.75 mm) within the RESOLUTE Global Clinical Trial Program over long-term (three-year) follow-up.
Methods
CLINICAL STUDIES
This pooled analysis of the RESOLUTE Global Clinical Trial Program includes the following clinical studies: RESOLUTE Trial, RESOLUTE All Comers Randomized Controlled Trial, RESOLUTE US Trial, RESOLUTE US 38 mm Trial, RESOLUTE Japan Trial, RESOLUTE Japan Small Vessel Study, RESOLUTE Asia Trial, and RESOLUTE China Randomized Controlled Trial7,10-15. Although the lesion criteria for study inclusion were assessed by site operators, all studies utilised an independent core laboratory for baseline lesion measurements, including RVD. This analysis does not include the RESOLUTE International Registry or the RESOLUTE China Registry as both registries did not utilise core laboratory assessment of baseline lesion characteristics. Furthermore, this analysis includes only patients treated with R-ZES at the index procedure.
The clinical design of these studies has been reported previously. Briefly, the RESOLUTE Trial7 was a first-in-man study of R-ZES conducted in Australia and New Zealand, RESOLUTE All Comers10 was a randomised controlled trial of R-ZES and the XIENCE V® everolimus-eluting stent (EES) (Abbott Vascular, Santa Clara, CA, USA) in a real-world all-comer population in Europe, and RESOLUTE US11 and RESOLUTE Japan13 enrolled patients implanted with R-ZES with the instructions for use in the respective countries. The RESOLUTE US 38 mm12 and RESOLUTE Asia 38 mm cohorts14 included patients with a long coronary lesion implanted with a 38 mm-long R-ZES. The RESOLUTE Japan Small Vessel Study13 included patients with R-ZES in small coronary arteries, and the RESOLUTE Asia dual-vessel cohort14 included patients with R-ZES for multivessel treatment. The RESOLUTE China Randomized Controlled Trial was a randomised controlled trial of R-ZES and the TAXUS™ paclitaxel-eluting stent (Boston Scientific, Marlborough, MA, USA) in a real-world all-comer population in China. All patients provided written informed consent, and the protocols were approved by the institutional review boards or ethics committees at all sites.
Dual antiplatelet therapy before implantation included aspirin 75 mg daily and clopidogrel either 75 mg daily or a ≥300 mg loading dose. After the procedure, patients were required to continue daily aspirin ≥75 mg indefinitely and daily clopidogrel 75 mg for a minimum of six months and up to 12 months in patients who were not at high risk of bleeding.
DEFINITIONS
Patients treated for a lesion during the index procedure with a core laboratory-measured RVD ≤2.25 mm were defined as the XS cohort, while those with an RVD >2.25 mm to ≤2.75 mm were defined as the SV cohort. Patients with multiple lesions treated during the index procedure with those lesions categorised as both XS and SV were analysed in the XS cohort.
Target lesion failure (TLF) was defined as cardiac death, target vessel myocardial infarction, and clinically driven TLR. Major adverse cardiac events (MACE) were defined as all-cause death, myocardial infarction, emergent coronary artery bypass surgery, or repeat clinically indicated target lesion percutaneous or surgical revascularisation. Target vessel failure (TVF) was defined as cardiac death, myocardial infarction, or clinically driven TVR by percutaneous or surgical methods. Clinically driven TLR (and TVR) was defined as revascularisation at the target lesion (and target vessel) associated with positive functional ischaemia study or ischaemic symptoms AND an angiographic minimal lumen diameter stenosis ≥50% by quantitative coronary angiography, or revascularisation of a target lesion with diameter stenosis ≥70% by quantitative coronary angiography without either angina or a positive functional study. Stent thrombosis was defined as Academic Research Consortium (ARC) definite or probable stent thrombosis16. “Complex patients”, as defined previously10, included patients treated with the index stent who had any of the following baseline lesion characteristics: bifurcation lesion, bypass graft, in-stent restenosis, unprotected left main, more than two vessels requiring treatment with R-ZES, creatinine >140 µmol/L, lesion length greater than 27 mm, more than one lesion treated per vessel, a lesion with thrombus or total occlusion (defined as pre-procedure Thrombolysis In Myocardial Infarction 0), as well as patients who presented with acute myocardial infarction of less than 72 hours duration, or left ventricular ejection fraction less than 30%.
STATISTICAL ANALYSIS
Continuous parameters are presented as mean±standard deviation and compared using the t-test or Wilcoxon rank-sum test as appropriate. Nominal parameters are presented as percentages and compared using Fisher’s exact test. The incidence rates were calculated using the Kaplan-Meier method and compared using the log-rank test.
Because patients with XS and SV coronary lesions at the index treatment site had different baseline characteristics, propensity score adjustment was used with a Cox regression model when comparing these two cohorts using the following baseline covariates: age, sex, history of smoking, current smoking, prior PCI, history of hyperlipidaemia, diabetes mellitus, insulin-dependent diabetes mellitus, hypertension, prior myocardial infarction, premature coronary artery disease in a first-degree relative, prior coronary artery bypass graft (CABG) surgery, reason for revascularisation, vessel location, American Heart Association/American College of Cardiology lesion class B2/C, moderate/severe calcification, lesion located at a bend ≥45 degrees, Thrombolysis In Myocardial Infarction 3 flow, complex patients (as defined above), pre-minimum lumen diameter, pre-diameter stenosis, lesion length, lesions treated per patient, number of stents per patient, multivessel treatment, and total stent length per patient.
P-values <0.05 were considered statistically significant. Analyses were performed using SAS software, version 9.1 or later (SAS Institute, Cary, NC, USA).
Results
Of 2,141 patients from the RESOLUTE Global Clinical Trial Program included in this analysis, 1,304 had RVD >2.25 mm and ≤2.75 mm (SV) and 837 had RVD ≤2.25 mm (XS). The baseline demographic and angiographic characteristics in each group are summarised in Table 1 and Table 2. Both mean RVD and minimal lumen diameter were significantly smaller in the XS than in the SV group (2.2±0.4 mm vs. 2.6±0.3 mm, p<0.001 and 0.8±0.4 mm vs. 0.9±0.4 mm, p<0.001, respectively). Patients with XS vessels were more likely to have hyperlipidaemia, prior PCI, and in-stent restenosis. However, the reason for revascularisation and rates of diabetes or prior CABG did not differ between groups.
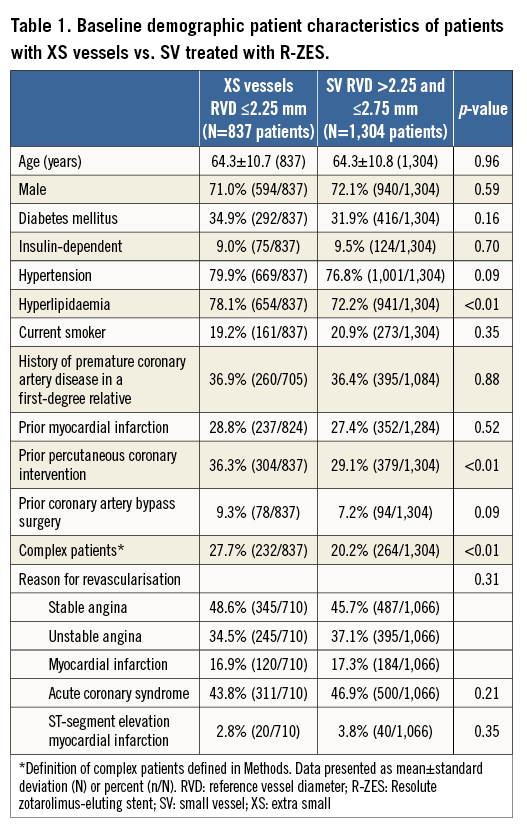
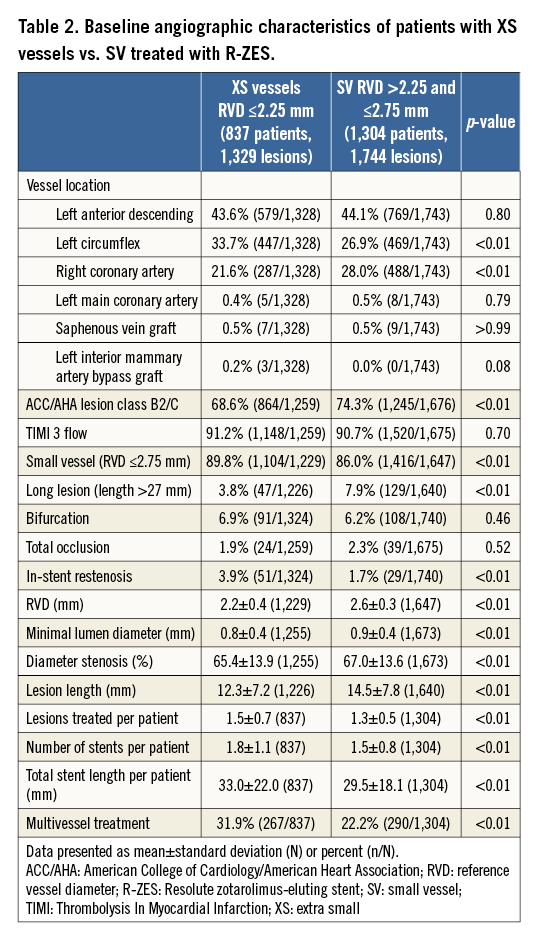
Patients with XS vessels tended to have shorter target lesion lengths (12.3±7.2 mm vs. 14.5±7.8 mm, p<0.001) but a greater degree of multivessel involvement (31.9% vs. 22.2%, p<0.001). A greater number of stents (1.8±1.1 vs. 1.5±0.8, p<0.001) and greater length of stents (33.0±22.0 vs. 29.5±18.1 mm, p<0.001) were placed in patients with XS vessels than SV. The XS cohort had more frequent left circumflex and less frequent right coronary involvement than the SV group, with no significant differences in left anterior descending, left main, or bypass graft target locations (Table 1).
UNADJUSTED CLINICAL OUTCOMES
Table 3 provides the three-year cumulative incidence of clinical outcomes. The three-year cumulative incidence of MACE was greater in the XS compared with the SV cohort (15.4% vs. 11.5%, p=0.01) (Figure 1A). These results were largely driven by higher rates of TLR (6.9% vs. 4.5%, p=0.02) (Figure 1B) and non-target lesion TVR (5.8% vs. 4.2%, p=0.07) in the XS group, which mirrored higher rates of TVR and TLF (Figure 1C). Rates of all-cause death (2.4% vs. 1.9%, p=0.50) and myocardial infarction (4.4% vs. 3.6%, p=0.36) were, however, similar across groups. Dual antiplatelet usage in XS and SV was 95% vs. 96% at 30 days, 89% vs. 90% at one year, and 42% vs. 43% at three years, respectively, (p=NS for all). The rate of definite or probable stent thrombosis was low without significant differences between XS and SV (1.2% vs. 0.6%, p=0.09) (Figure 1D), although numerically higher in the XS cohort. Most stent thromboses were definite (0.8% vs. 0.4%, p=0.17) and roughly half occurred in the first 30 days.
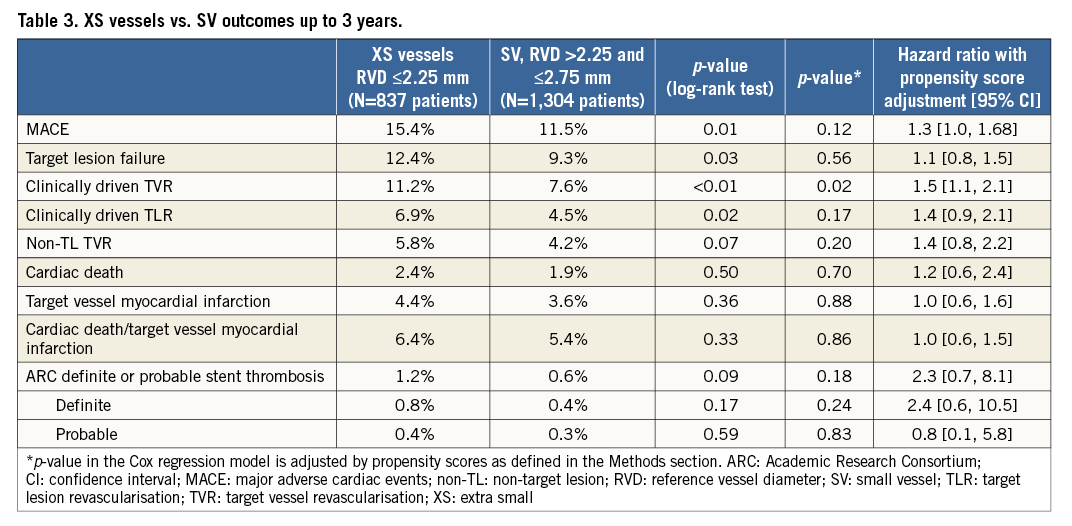
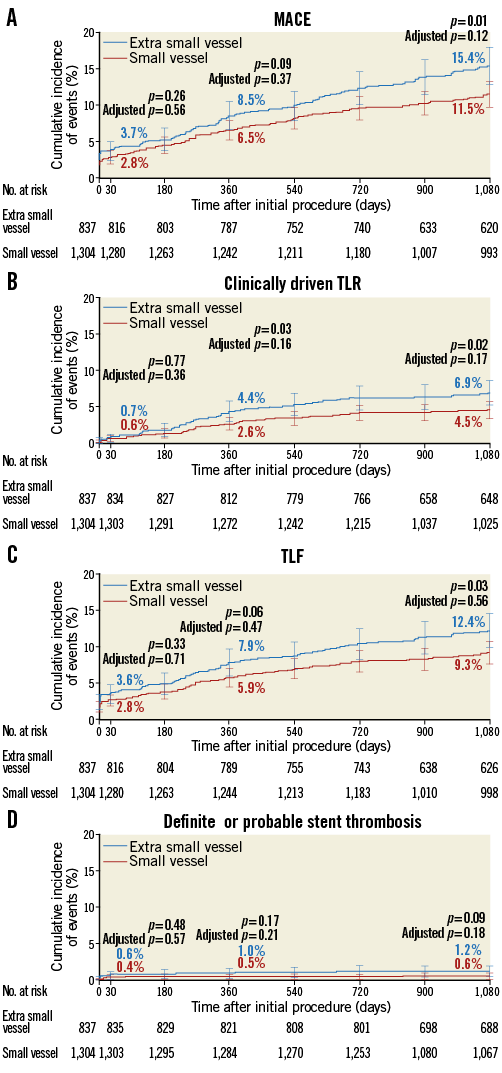
Figure 1. Three-year cumulative incidence of events in patients treated with a Resolute zotarolimus-eluting stent in an extra-small vessel (≤2.25 mm diameter) or small vessel (>2.25 and ≤2.75 mm diameter). A) Major adverse cardiac events. B) Clinically driven target lesion revascularisation. C) Target lesion failure. D) Academic Research Consortium definite or probable stent thrombosis.
PROPENSITY-ADJUSTED CLINICAL OUTCOMES
Table 3 shows the components of MACE at three years for each group after propensity score adjustment to take into account the differences in baseline demographics between patients with XS and SV. After adjustment, there were no significant differences in MACE, TLR, and TLF. The hazard of TVR, however, remained higher in the XS group (hazard ratio [95% confidence interval] 1.5 [1.1, 2.1], p=0.02). Differences in stent thrombosis, death, and myocardial infarction remained non-significant after adjustment.
DIABETIC PATIENTS
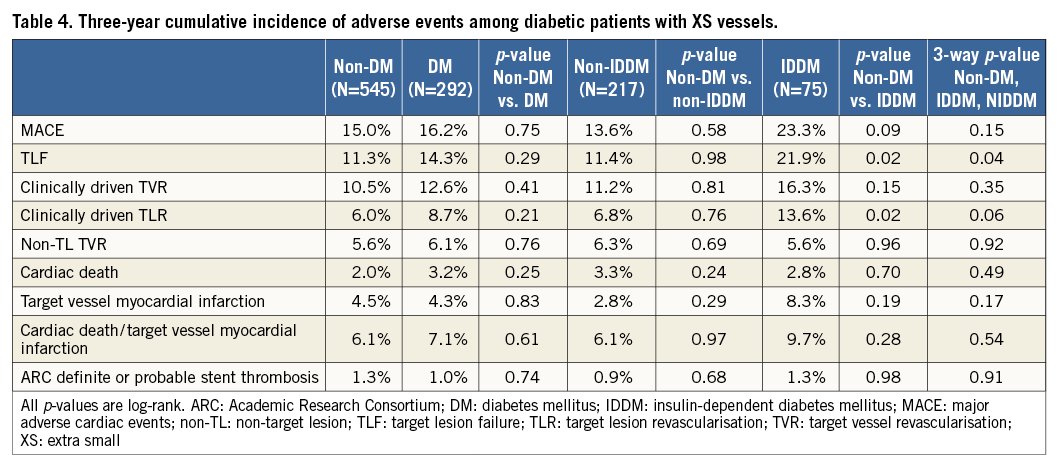
In the XS group, 292 patients had diabetes mellitus (DM), and 545 were non-diabetic. There were no differences in overall MACE events or ARC definite or probable stent thrombosis between diabetic and non-diabetic patients (16.2% vs. 15.0%, p=0.751, and 1.0% vs. 1.3%, p=0.739, respectively) (Table 4). When comparing non-diabetics to the subgroup of diabetics taking insulin (n=75), there was an increase in both clinically driven TLR and TLF among XS patients with DM who took insulin (13.6% vs. 6.0%, p=0.02, and 21.9% vs. 11.3%, p=0.02, respectively) with no differences in clinically driven TVR (16.3% vs. 10.5%, p=0.15) or non-TL TVR (5.6% vs. 5.6%, p=0.96).
Discussion
In this large-scale analysis of patients with SV treated with R-ZES, the long-term lesion-specific results among patients with XS (≤2.25 mm) vessels treated with the R-ZES were not significantly different from those among patients with vessel sizes of >2.25 to 2.75 mm after propensity adjustment. However, specific patients with XS vessels (e.g., those with insulin-dependent diabetes) remain at high risk for TLR, even with DES. In addition, given that XS vessel disease is often a manifestation of a diffuse disease process, it is important to be aware of higher rates of overall TVR (attributable both to the target lesion as well as non-target segments) that can occur with treatment of XS vessels.
Small-vessel intervention has historically suffered from a relatively less tolerated late lumen loss compared with intervention in larger vessels, resulting in higher rates of restenosis and repeat revascularisation1,2,17. However, the introduction of first-generation DES significantly reduced rates of revascularisation in small vessels, and even coincided with a rise in the incidence of SV intervention4,18,19.
Second-generation DES have reduced overall adverse events in SV even further. One-year TLR in a registry was 3.8% in <2.5 mm vessels treated with a single XIENCE V EES, slightly higher but not statistically different from a 3.0% TLR rate in vessels >2.5 mm5. Compared with PES, EES also had lower two-year rates of cardiac death, myocardial infarction, and TLR in patients with SV; furthermore, this benefit of EES over PES appeared to be most prominent in those patients with small RVD or long lesion length3.
In a study of 5,014 patients, the R-ZES had previously been shown to have comparable performance in small (<2.5 mm) and larger vessels (>2.5 mm), with two-year TLR rates of 5.3% and 4.4% (p=0.34), respectively, and no significant differences in overall MACE, clinically driven TVR, or stent thrombosis9. In a propensity-adjusted analysis, the present study extends those findings to even smaller vessels. Over a three-year follow-up period, the rate of TLR was 6.9% in vessels ≤2.25 mm and statistically similar to the 4.5% rate in vessels >2.25 to ≤2.75 mm.
Unlike the prior analysis, there were persistent differences in TVR (11.2% vs. 7.5%, p=0.022), and unadjusted rates of TLR and TLF were higher in the XS group. These findings may reflect the fact that the XS group had higher baseline risk factors, including a higher proportion of “complex patients”, hyperlipidaemia, and multivessel treatment as well as increased revascularisation of the non-target lesion in XS vessels. These differences, in both randomised and non-randomised studies, provide the rationale for reporting an adjusted analysis.
Similar to prior studies of SV stenting, there was a trend towards a higher rate of three-year ARC definite or probable stent thrombosis (1.2% vs. 0.6%, p=0.18), although this did not reach statistical significance. These rates are comparable to those reported in SV in the pooled analysis of R-ZES (0.8% at two years) and EES (1.1% to 1.4% at two years)3,9. The ARC definite or probable stent thrombosis rate at one year in XS vessels (1.0%) is also similar to that observed with EES in the SPIRIT Small Vessel Trial (with the 2.25 mm EES stent) (1.5%)20.
While studies of older stents suggested significant differences depending on diabetic status, overall outcomes were similar in diabetics compared with non-diabetics, a result in line with prior studies on SV stenting using second-generation stents5,9. On the other hand, the subgroup of insulin-dependent diabetics had higher rates of TLR and TVF than the subgroup of non-diabetics. Although this may truly reflect a higher risk of stenting XS vessels in this population, it may also represent a spurious finding given the small number (n=75) of patients in this subgroup along with likely misclassification in distinguishing “insulin-dependent” from “non-insulin-dependent” diabetics. Larger studies are needed to confirm or refute this result.
Limitations
This study included a heterogeneous set of patients across eight trials with different inclusion criteria, protocols, and trial committees, although endpoint definitions and data collection were homogenous across the Global Clinical Trial Program. Attempts were made to limit confounding using propensity matching in a multivariable analysis; however, residual confounding of unmeasured factors cannot be entirely eliminated by this approach.
The choice of reference vessel groups in this study coincides with available stent platform sizes; however, prior studies have defined “small vessels” variably as <3 mm, <2.75 mm, <2.65 mm, and <2.5 mm, which somewhat limits interpretation of these results in the context of others3,9,21. To our knowledge, however, no other studies of second-generation DES have subdivided populations to evaluate the outcomes of stenting vessels ≤2.25 mm with follow-up >2 years. The decision to stent XS vessels should be made on an individualised basis. Unfortunately, the specific clinical reasons for revascularisation of these vessels and lesions were incompletely captured on the case report forms for the included studies (missing in approximately 15% of cases); however, all patients included in the trials for this analysis met the standards of the protocol. Despite the use of an advanced stent design, these results suggest that there are persistent differences between stenting SV and XS vessels, in particular higher rates of TLR in insulin-treated diabetic patients. Additionally, while it is of clinical relevance to identify what proportion of these treated vessels went on to total occlusion, unfortunately the proportion of vessels occluded at follow-up was not systematically ascertained within these trials.
Conclusions
Although the long-term, lesion-specific results among patients with XS (≤2.25 mm) vessels treated with the R-ZES were not significantly different from those among patients with vessel sizes of >2.25 to 2.75 mm, particularly after propensity adjustment, specific patients with XS vessels (e.g., those with insulin-dependent diabetes) may remain at high risk for TLR.
| Impact on daily practice Although newer-generation drug-eluting stents suppress late loss and reduce clinical restenosis, the performance of these devices within the smallest coronary vessels (with less “tolerated” late loss) is incompletely understood. In this analysis from the RESOLUTE Global Clinical Trial Program, long-term (three-year) lesion-specific results among patients with vessel size ≤2.25 mm treated with the R-ZES were not significantly different from those among patients with vessels >2.25 to 2.75 mm. However, TVR rates were higher in patients with the smallest vessels, indicative of a more diffuse disease process. |
Funding
This study and the RESOLUTE Global Clinical Trial Program are funded by Medtronic.
Conflict of interest statement
M. Parikh is an advisory board member for Medtronic, Abbott Vascular, and Philips, and a member of the speaker’s bureau for Boston Scientific and Cardiovascular Systems, Inc. M. Leon is an advisory board member for Medtronic. A. Kirtane receives institutional research grants from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, St. Jude Medical, Medinol, and Eli Lilly. The other authors have no conflicts of interest to declare.

