
Abstract
Aims: Our aim was to investigate whether long-term (three-year) clinical outcomes after multivessel treatment with the Resolute zotarolimus-eluting stent (R-ZES) were similar to single-vessel treatment.
Methods and results: The RESOLUTE Global Clinical Trial Program enrolled 7,618 patients, of whom 1,562 underwent multivessel and 6,053 single-vessel treatment with the R-ZES. Patients in the multivessel group were more likely to have complex lesions (58% vs. 44%, p<0.001). Clinical outcomes were compared using a Cox regression model adjusted by propensity score to account for differences in baseline characteristics. Compared with single-vessel treatment, multivessel treatment was associated with more complex anatomy and longer mean total stent length (57.8±28.6 vs. 26.7±15.2 mm, p<0.001). At three years, the cumulative incidence of target lesion failure was similar in patients with multivessel and single-vessel treatment (11.0% vs. 9.1%, adjusted p=0.986), as was the incidence of cardiac death or target vessel myocardial infarction (6.7% vs. 5.7%, adjusted p=0.793), the incidence of clinically driven target lesion revascularisation (5.1% vs. 4.4%, adjusted p=0.904), and the incidence of Academic Research Consortium definite or probable stent thrombosis (1.2% vs. 0.9%, adjusted p=0.544).
Conclusions: Multivessel treatment with R-ZES provided good long-term clinical outcomes that were comparable to those achieved with single-vessel stenting, supporting the efficacy and safety of R-ZES in patients in this setting.
Introduction
Currently, coronary artery bypass graft surgery (CABG) is the preferred revascularisation strategy for patients with complex multivessel coronary artery disease (CAD), based on the results of the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) and Comparison of Two Treatments for Multivessel Coronary Artery Disease in Individuals With Diabetes (FREEDOM) trials1,2. However, both studies used first-generation drug-eluting stents (DES) that have worse outcomes than second-generation DES3,4. Furthermore, limited data are available on the outcomes of current-generation DES in multivessel stenting5,6.
The current-generation Resolute™ zotarolimus-eluting stent (R-ZES) (Medtronic, Minneapolis, MN, USA) has shown low rates of long-term adverse clinical outcomes among unselected, real-world populations, and similar outcomes to the XIENCE V® (Abbott Vascular, Santa Clara, CA, USA) everolimus-eluting stent (EES)7-9. We compared outcomes after multivessel vs. single-vessel treatment with the R-ZES across the RESOLUTE Global Clinical Trial Program. We also examined outcomes among patients with multivessel treatment based on mean total stent length, number of stents implanted per vessel, and diabetic status.
Methods
The RESOLUTE Global Clinical Trial Program includes 7,618 patients treated with R-ZES across 10 studies (Table 1). The methods for these studies have been described previously. Briefly, the RESOLUTE trial10,11 was a first-in-man R-ZES study conducted in Australia and New Zealand. RESOLUTE All Comers12-14 was a randomised controlled trial comparing outcomes with R-ZES to outcomes with the EES in a real-world all-comer population in Europe. RESOLUTE International8,15 was a worldwide registry treating patients in a real-world all-comer population with R-ZES; both RESOLUTE US16,17 and RESOLUTE Japan18 enrolled patients in line with the respective country’s instructions for use. RESOLUTE US 38 mm19 was a substudy of RESOLUTE US that included patients who were appropriate for a 38 mm R-ZES stent. RESOLUTE Asia included two cohorts, the RESOLUTE Asia Dual Vessel cohort, which included patients with at least two lesions in at least two different vessels20, and the RESOLUTE Asia 38 mm cohort19,20. Both the RESOLUTE China Randomized Controlled trial3 and the RESOLUTE China registry21 were conducted in China in real-world populations. Among the 7,618 patients enrolled in the RESOLUTE Global Clinical Trial Program, most patients (72%) were enrolled in studies that enrolled all-comer populations. All patients provided written informed consent, and the protocols were approved by the institutional review board or ethics committee at each site.
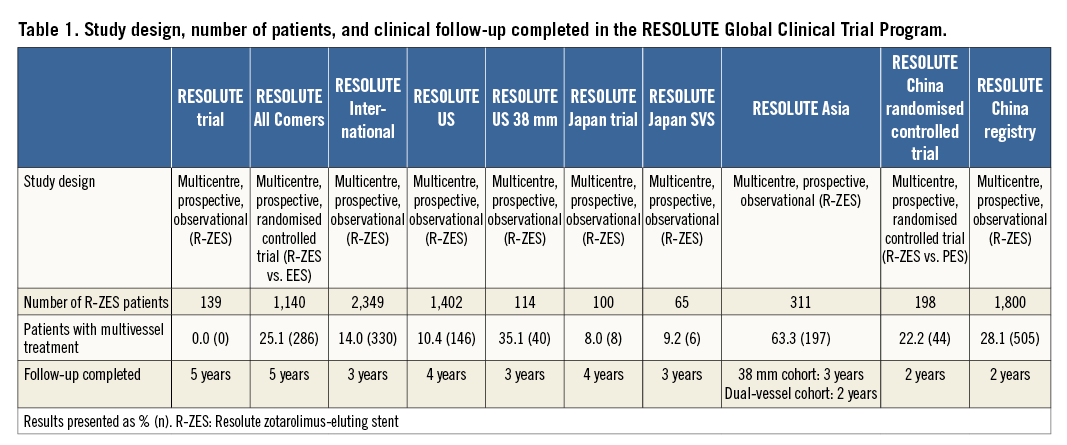
Baseline lesion characteristics were determined by an independent core laboratory in all studies except RESOLUTE International and the RESOLUTE China Registry (that reported results based on visual estimation). Follow-up was carried out in the clinic or by telephone. Target lesion failure was defined as a composite of cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularisation (TLR). Target vessel myocardial infarctions were defined as all myocardial infarctions not clearly attributable to a non-target vessel. All myocardial infarctions were adjudicated according to the extended historical definition22. The studies contributing to this analysis used consistent endpoint definitions. Study-specific clinical event committees whose members were not directly involved in the study applied these definitions to all suspected events. Data safety monitoring boards were composed of non-interventional and interventional cardiologists not directly involved in the study. Clinical event committees and data safety monitoring boards were coordinated by independent academic research organisations.
Statistical analysis
The analysis on multivessel vs. single-vessel treatment with R-ZES in the RESOLUTE Global Clinical Trial Program was a post hoc analysis. Patients with two or more vessels stented during the index procedure were included in the multivessel cohort and were compared with patients with a single vessel stented during the index procedure. Given the differences in baseline characteristics between single-vessel and multivessel treatment, clinical outcomes between these two groups were compared using a Cox regression with propensity score adjustment. Specifically, propensity scores were calculated using the following baseline covariates: age, sex, history of smoking, current smoking, prior PCI, hyperlipidaemia, diabetes mellitus, insulin-dependent diabetes mellitus, history of hypertension, prior myocardial infarction, premature CAD in a first-degree relative, prior CABG, reason for revascularisation, vessel location, American Heart Association/American College of Cardiology B2/C lesion class, moderate or severe calcification, lesion bend less than or equal to 45°, Thrombolysis In Myocardial Infarction (TIMI) 3 flow, pre-procedure reference vessel diameter, pre-procedure minimal lumen diameter, pre-procedure diameter stenosis, lesion length, number of lesions treated per patient, number of stents per patient, mean total stent length per patient, and “complex patients”. (For pre-procedure reference vessel diameter, minimal lumen diameter, diameter stenosis, if a patient had more than one lesion treated, the smallest diameter was used). “Complex patients” was defined as treatment of any of the following: bifurcation lesion, bypass graft, in-stent restenosis, acute myocardial infarction (<72 hours), left ventricular ejection fraction <30%, unprotected left main artery, >2 vessels stented, renal insufficiency or failure (defined as creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, or lesion with thrombus or total occlusion (defined as pre-procedure TIMI 0 flow). Then, for each clinical outcome, a Cox regression model was performed with the outcome as the dependent variable, and number of vessels (single vs. multivessel) and propensity score as independent variables.
The cumulative incidence of events at three years was calculated using the Kaplan-Meier method and compared between the two study groups using the log-rank test. P-values <0.05 were considered statistically significant. Analyses were performed using SAS software version 9.1 or later (SAS Institute, Cary, NC, USA).
Results
Among the 7,618 patients treated with R-ZES in the RESOLUTE Global Clinical Trial Program, 1,562 patients (3,445 lesions) underwent multivessel treatment and 6,053 patients (6,741 lesions) single-vessel treatment with R-ZES. The baseline patient characteristics differed between the two groups (Table 2). Compared with patients who underwent single-vessel stenting, patients who received multivessel stenting often had more complex baseline characteristics, including a higher prevalence of diabetes mellitus, and acute coronary syndrome, but had a lower prevalence of hyperlipidaemia, family history of CAD, prior PCI, and prior CABG. Compared with single-vessel treatment, multivessel treatment was performed in more complex lesions, including more left main and bifurcation lesions and smaller diameter reference vessels (Table 3). Multivessel treatment involved more lesions treated per patient, more stents implanted per patient, and a greater mean total stent length per patient (Table 3).
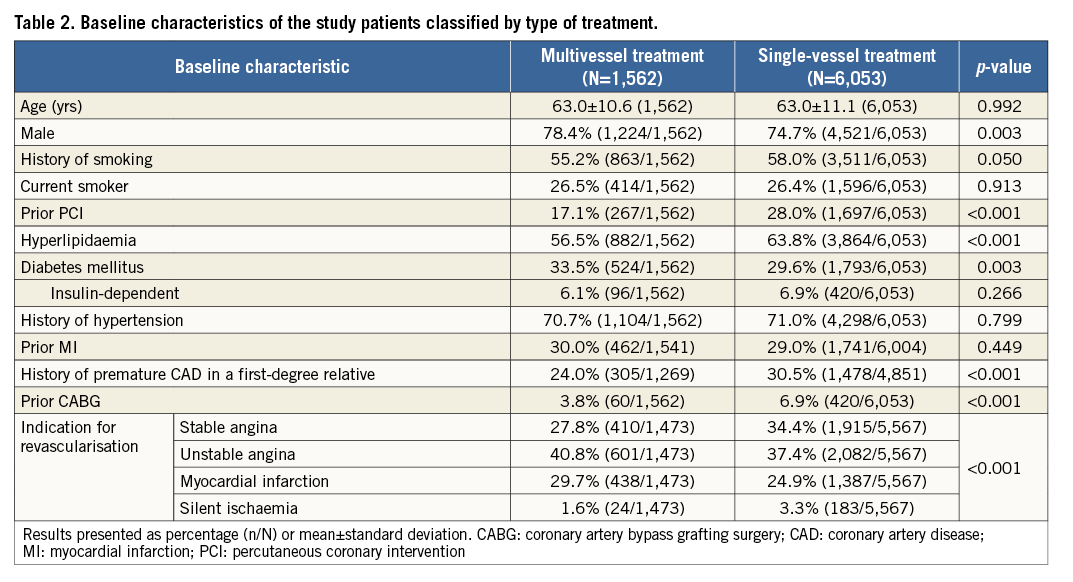
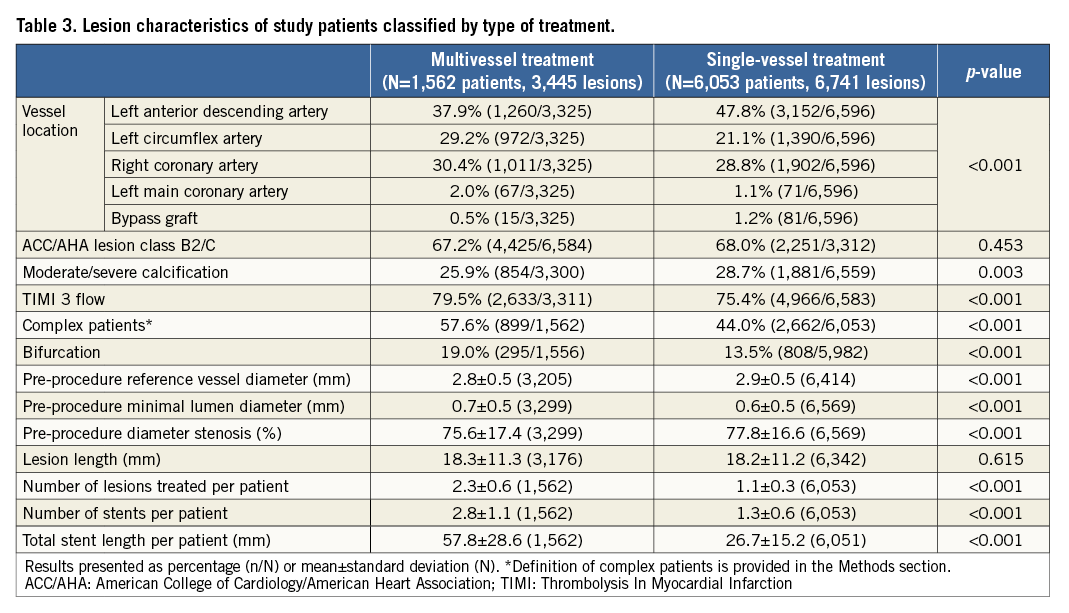
Table 4 provides the three-year cumulative incidence of events. As compared with single-vessel treatment, multivessel treatment resulted in a similar incidence of target lesion failure (11.0% vs. 9.1%, adjusted p=0.986) (Figure 1A), or its components cardiac death or target vessel myocardial infarction (6.7% vs. 5.7%, adjusted p=0.793) (Figure 1B), and clinically driven TLR (5.1% vs. 4.4%, adjusted p=0.904) (Figure 1C). All-cause mortality was significantly lower in patients who underwent multivessel treatment, but there was no difference in cardiac death. Administration of dual antiplatelet therapy in patients with multivessel vs. single-vessel treatment was above 90% at 12 months (91.5% and 90.8%, p=0.453) and below 50% at 24 months (48.5% and 47.2%, p=0.381) and 36 months (32.5% and 38.8%, p<0.001). The three-year cumulative incidence of Academic Research Consortium (ARC) definite or probable stent thrombosis in patients with multivessel or single-vessel treatment was 1.2% vs. 0.9% (adjusted p=0.544) (Figure 1D).
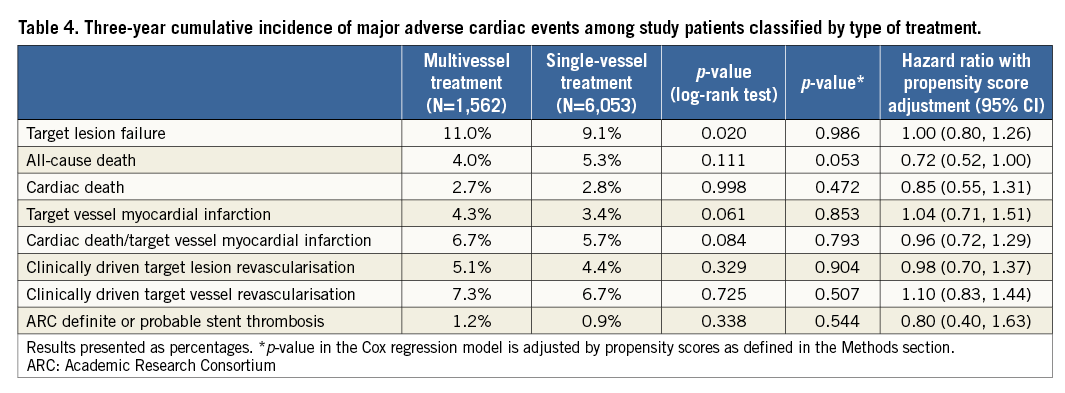
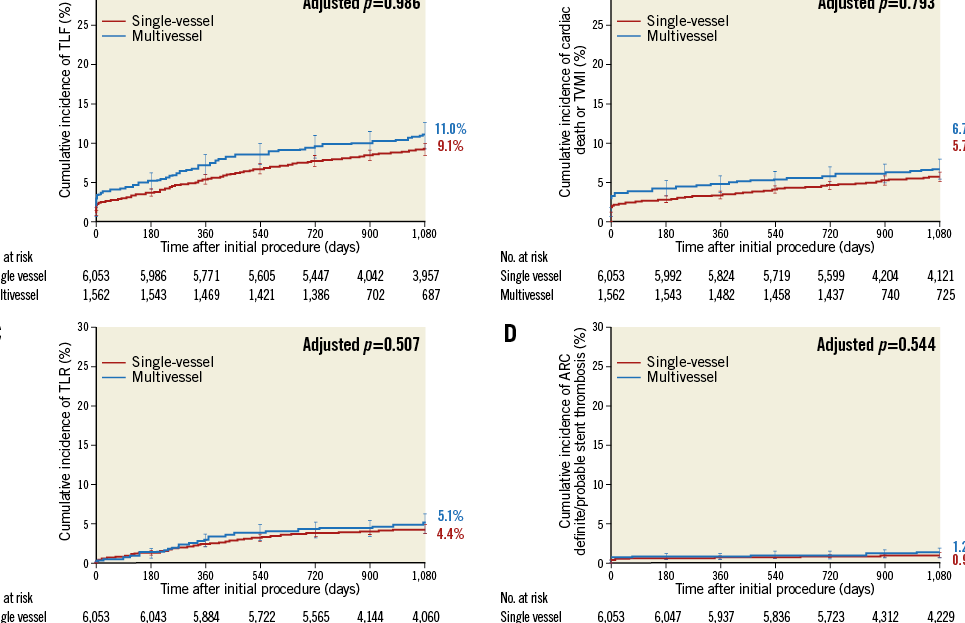
Figure 1. Three-year cumulative incidence of target lesion failure (A), cardiac death or target vessel myocardial infarction (B), clinically driven target lesion revascularisation (C), and stent thrombosis (D) in multivessel vs. single-vessel treatment with the Resolute zotarolimus-eluting stent.
Multivessel treatment required longer total stent length. Among patients with multivessel treatment, when analysing total stent length by tertiles, the total stent length was 29.2±6.5 mm in the first tertile of patients (n=514), 46.88±4.86 mm in the second tertile (n=532), and 79.3±24.1 mm in the third tertile (n=514). Despite the long total stent length, the incidence of clinical adverse events was low. The three-year cumulative incidence of target lesion failure in the first, second, and third tertiles was 7.9%, 11.6%, and 13.4% (three-way p=0.015) (Figure 2), respectively; cardiac death/target vessel myocardial infarction, 4.7%, 6.5%, and 8.7%, (three-way p=0.038); clinically driven TLR, 3.2%, 5.9%, and 6.2% (three-way p=0.090); and ARC definite/probable stent thrombosis, 1.0%, 0.6%, and 2.2% (three-way p=0.022).
Among patients with multivessel treatment, 33% required >1 R-ZES stent in each target vessel. When compared with patients who received only one R-ZES per vessel in the multivessel group, patients with >1 R-ZES implanted in at least one vessel had a similar three-year cumulative incidence of target lesion failure (11.9% vs. 10.8%, p=0.549) (Figure 2) or its components cardiac death or target vessel myocardial infarction (8.0% vs. 6.2%, p=0.227) and clinically driven TLR (5.3% vs. 5.0%, p=0.224). The ARC definite/probable stent thrombosis rate was, however, higher in patients with >1 stent per vessel (2.6% vs. 0.8%, p=0.007). Although the three-year cumulative incidence of stent thrombosis was higher among multivessel treatment patients with at least one vessel with >1 R-ZES, the mean total stent length in these patients was 77.7±29.1 mm (among these same patients who also had a stent thrombosis, mean total stent length was 84.2±53.1 mm).
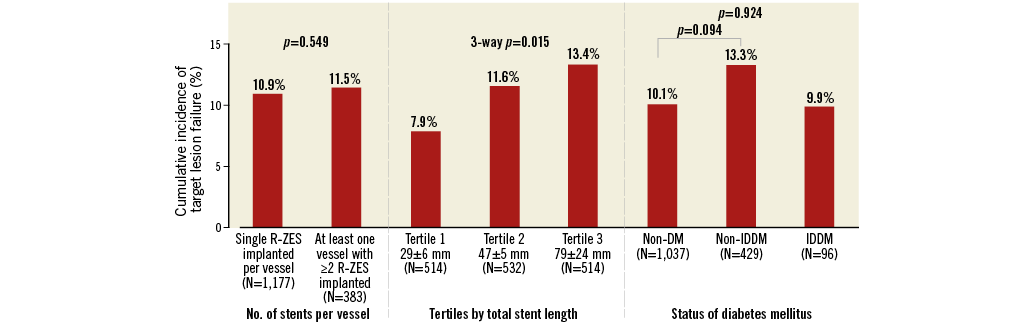
Figure 2. Three-year cumulative incidence of target lesion failure among patients with multivessel stent implantation with the Resolute zotarolimus-eluting stent. DM: diabetes mellitus; IDDM: insulin-dependent diabetes mellitus; R-ZES: Resolute zotarolimus-eluting stent
In the multivessel treatment group, patients without (n=1,037) and with (n=429) non-insulin-dependent diabetes mellitus had a similar three-year cumulative incidence of target lesion failure (10.1% vs. 13.3%, p=0.094) (Figure 2, Figure 3A), cardiac death/target vessel myocardial infarction (6.1% vs. 7.8%, p=0.186) (Figure 3B), clinically driven TLR (4.8% vs. 6.1%, p=0.438) (Figure 3C), and ARC definite/probable stent thrombosis (1.4% vs. 1.1%, p=0.382) (Figure 3D). Also, patients with insulin-dependent diabetes mellitus had similar outcomes to non-diabetic patients (Figure 3A-Figure 3D).
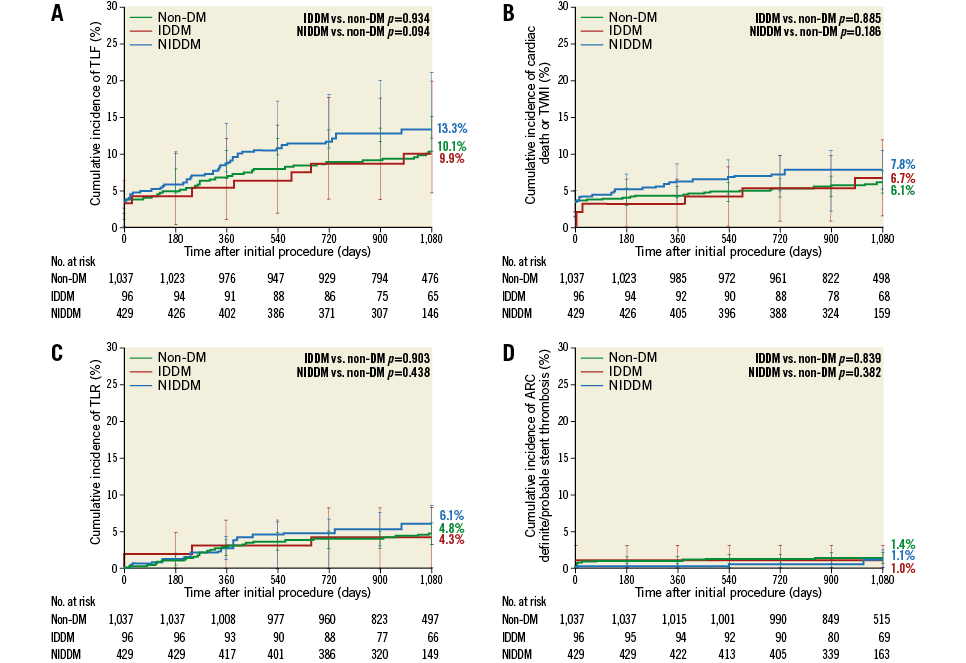
Figure 3. Adjusted three-year cumulative incidence of target lesion failure (A), cardiac death or target vessel myocardial infarction (B), clinically driven target lesion revascularisation (C), and stent thrombosis (D) among study multivessel patients classified according to the patient’s diabetic status.
Discussion
Patients who underwent multivessel treatment (n=1,562) with R-ZES in the RESOLUTE Global Clinical Trial Program had a low three-year incidence of adverse clinical outcomes, including target lesion failure, cardiac death, target vessel myocardial infarction, TLR, and ARC definite or probable stent thrombosis. Although the rate of events significantly increased in parallel to stent length, the overall incidence of these outcomes remained low and was similar to that observed after single-vessel stenting (n=6,053) after propensity score adjustment, supporting the use of R-ZES to treat multivessel CAD. In addition, before propensity score adjustment, there were no significant differences between the two cohorts in terms of the three-year cumulative incidence of cardiac death, target vessel myocardial infarction, or clinically driven TLR. However, the composite three-year cumulative incidence of target lesion failure was higher in multivessel treatment before propensity score adjustment but not after (11.0% vs. 9.1%, p=0.020, adjusted p=0.986, HR 1.00 [95% CI: 0.80-1.26]). Use of dual antiplatelet therapy at three years was high in our analysis both in patients who received multivessel and in those who received single-vessel stenting (32.5% and 38.8%, respectively, p<0.001), and was probably influenced by geographic variation.
Clinical outcomes among patients who underwent multivessel stenting with R-ZES in our analysis were similar to those observed with other second-generation DES. In a pooled analysis of SPIRIT III and SPIRIT IV, among patients who underwent multivessel stenting with EES (n=511), the one-year rate of target lesion failure was 6.0%5, similar to that observed with R-ZES in our analysis (7.2%). Additionally, in the EXecutive RCT: Evaluating XIENCE V in a Multi Vessel Disease (EXECUTIVE) trial, the one-year incidence of clinically driven TLR after multivessel PCI with EES was 6.1%6, which is similar to that observed in our analysis with R-ZES (2.9%). The three-year cumulative incidence of stent thrombosis among patients who underwent multivessel and single-vessel PCI with R-ZES in our study was low at 1.2% and 0.9%, respectively (p=0.544). Post hoc analysis of the SPIRIT III trial patients who underwent two-vessel PCI with the EES showed a two-year ARC definite/probable stent thrombosis rate of approximately 4% (as compared with ~1% among patients who underwent single-vessel PCI)23.
As anticipated, when patients in our study were classified by mean total stent length, the third tertile had a higher risk for subsequent events. Total stent length of DES has been shown to be associated with an increased risk for stent thrombosis9,24, in-stent restenosis25, and TLR24, possibly because of stent underexpansion over a long artery segment with decreasing diameter from proximal to distal. In our study, mean total stent length among the third tertile was 79.3±24.1 mm, yet the three-year incidence of clinically driven TLR in this patient subgroup was only 6.2%. In the j-Cypher Registry, the mean stent length among the fourth quartile of patients was 51.7±15.7 mm (n=2,184) and was associated with a three-year rate of TLR of 21% (as compared with 7.5% in the first quartile, which had a mean total stent length of 17.4±1.6 mm [n=4,647])24.
In patients who underwent multivessel stenting in our analysis, target lesion failure was similar among patients who received >1 R-ZES in at least one vessel as compared with those who received only one R-ZES implanted in each treated vessel. However, the three-year cumulative incidence of stent thrombosis was higher among patients who received >1 stent per vessel. Mean total stent length in these patients was 77.7±29.1 mm; therefore, these patients are mostly the same patients as those in the third tertile by mean total stent length. Farooq et al26 analysed outcomes in patients with and without overlapping stents in the RESOLUTE Global Clinical Trial Program and found no difference between those with single and those with multiple stents per treated vessel.
The TWENTE (The Real-World Endeavor Resolute Versus XIENCE V Drug-Eluting SteNt Study: Head-to-head Comparison of Clinical Outcome After Implantation of Second Generation Drug-eluting Stents in a Real World Scenario) trial randomised 1,391 real-world patients to R-ZES and XIENCE EES, and included a high proportion of patients (24%) who received PCI for multivessel treatment27. Target vessel failure was similar in both R-ZES and XIENCE arms at one year (8.2% vs. 8.1%, p=0.001 for non-inferiority)27 and three years (12.1% vs. 13.4%, p=0.50)28. At three years, target vessel failure was also numerically similar in all patients with dual-vessel stenting (main and side branch) treatment of bifurcation lesions (13.4%, n=82) and in patients without a bifurcation lesion (12.6%, n=1,021)29.
In the RESOLUTE Global Clinical Trial Program among patients with multivessel stenting with R-ZES, there was no difference in the three-year cumulative incidence of clinical outcomes between patients with diabetes mellitus and those without diabetes mellitus. These results are provocative, as diabetes mellitus continues to be a risk factor even with current-generation DES30. However, a previous analysis of the RESOLUTE Global Clinical Trial Program (including patients with single-vessel treatment with R-ZES) demonstrated no difference in outcomes between patients without diabetes mellitus and those with non-insulin-dependent diabetes mellitus; however, the higher-risk patients with insulin-dependent diabetes mellitus had a higher target lesion failure rate31. A similar finding was seen in the FREEDOM trial that compared outcomes of patients with multivessel CAD randomised to PCI or CABG, in which patients with insulin-dependent diabetes mellitus had a higher rate of major adverse cardiac events as compared with those not treated with insulin, independent of the revascularisation strategy32. More recently, the Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease (BEST) trial randomised patients with multivessel coronary artery disease to second-generation EES versus CABG and found CABG to be superior33.
In the light of the recent BEST trial, SYNTAX, and FREEDOM, CABG is still considered superior to PCI in subjects with triple-vessel disease. It may be that a more “targeted” treatment approach in haemodynamically significant vessels will result in further improvements in outcomes with PCI. The Comparison of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention and Coronary Artery Bypass Graft Surgery in Patients With Multivessel Coronary Artery Disease (FAME 3; ClinicalTrials.gov: NCT02100722) trial is enrolling patients with multivessel disease, who will be randomly assigned to fractional flow reserve-guided PCI with the R-ZES or CABG. The study will be particularly important to assess outcomes with the R-ZES in patients with three-vessel disease randomised to PCI or CABG, many of whom will probably have diabetes mellitus, including insulin-dependent diabetes mellitus.
Limitations
The present analysis is a post hoc analysis of trials not primarily intended to investigate outcomes in multivessel vs. single-vessel treatment or analyses specific to patients with multivessel treatment. However, dual-vessel treatment was a pre-specified analysis in several trials, and the majority of patients were enrolled in all-comer studies. The RESOLUTE Global Clinical Trial Program enrolled various populations from multiple countries, resulting in truly global results, potentially applicable to different clinical practices and ethnic groups. However, differences in study design may result in a possible source of heterogeneity between the single-vessel and multivessel cohorts. Patients in all-comer studies, often with more complex lesions, were more likely to undergo multivessel treatment. Additionally, a selection bias of patients included in registries cannot be ruled out. Propensity score adjustment was used to reduce the chance of such bias, but some bias could still exist.
Conclusions
Across the RESOLUTE Global Clinical Trial Program, multivessel stenting with R-ZES was associated with good clinical outcomes during long-term follow-up. The rate of events significantly increased in parallel to stent length; however, overall, results were similar to those observed for patients who underwent single-vessel treatment. These findings support the use of R-ZES in patients with multivessel disease, including those with complex CAD.
| Impact on daily practice Data to support the use of second-generation DES for the treatment of multivessel disease have been limited. This comparison of multivessel stenting with single-vessel stenting using R-ZES found that multivessel stenting provided comparable safety and efficacy to those observed with single-vessel stenting. However, long total stent length of DES has been associated with higher rates of clinical events, which were also observed in the current analysis. R-ZES offers an alternative to CABG in complex patients with multivessel disease. |
Guest Editor
This paper was guest edited by Clemens Von Birgelen, MD, PhD, FESC; Thoraxcentrum Twente, Medisch Spectrum Twente, Enschede, and University of Twente, Enschede, The Netherlands.
Acknowledgements
We thank Yun Peng and Minglei Liu for statistical support and Nicole Brilakis for editorial support (all from Medtronic).
Funding
Medtronic. Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique identifiers: RESOLUTE Clinical Trial, NCT00248079; RESOLUTE All Comers, NCT00617084; RESOLUTE International, NCT00752128; RESOLUTE US Clinical Trial, NCT00726453; RESOLUTE Japan Trial, NCT00927940; RESOLUTE Japan Small Vessel Study, NCT01150500; RESOLUTE Asia, NCT01132456; RESOLUTE China Randomized Controlled Trial, NCT01334268; and RESOLUTE China Registry, NCT01243749.
Conflict of interest statement
G. Manoharan has received consultant fees and honoraria from Medtronic, St. Jude Medical, and Boston Scientific. J. Belardi serves as an advisor and consultant to Medtronic. A. Yeung has served as an advisor to Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor reports institutional research grants from AstraZeneca, Biotronik, Boston Scientific, and Medtronic.

