Abstract
Aims: To provide clinical outcome data from everyday practice for the new generation Resolute zotarolimus-eluting stent (R-ZES).
Methods and results: Patients were eligible if placement of ≥1 R-ZES was intended. There were no restrictions on clinical indication, number of treated vessels, and lesion characteristics. The primary endpoint was the adjudicated cumulative 1-year incidence of cardiac death and target vessel myocardial infarction. Twenty-five per cent of the patients were randomly selected for monitoring. We recruited 2,349 patients with 3,147 lesions (1.6±1.0 stents per patient); 46.0% of patients had acute coronary syndrome, 30.5% were diabetic, and ≥1 complex criterion for stent placement was present in 67.5% of patients. One-year follow-up was complete in 97.9% of patients. The 1-year incidence of the primary endpoint was 4.3% (95% CI: 3.5% to 5.2%) and for ARC definite and probable stent thrombosis, 0.9% (0.5% to 1.3%). Clinically driven target lesion revascularisation and target lesion failure were 3.4% (2.7% to 4.3%) and 7.0% (6.0% to 8.2%), respectively. These findings were consistent across all lesion and patient subsets analysed. There were no significant differences in outcomes between monitored and unmonitored patients.
Conclusions: In everyday practice, the R-ZES performed similarly well as in the RESOLUTE All Comers randomised trial.
Introduction
The new-generation Resolute zotarolimus-eluting stent (R-ZES; Medtronic Inc., Minneapolis, MN, USA) has been developed with design features that favour deliverability and reduce late lumen loss compared with previous stent design, without compromising safety. The recently published RESOLUTE All Comers trial delineated the efficacy of the R-ZES.1 With 2,292 patients randomised, RESOLUTE All Comers demonstrated non-inferiority of the R-ZES compared with an everolimus-eluting stent (XIENCE V; Abbott Vascular, Santa Clara, CA, USA) with respect to the cumulative 1-year incidence of target lesion failure. Moreover, there were no significant differences between the two stents in terms of death from cardiac causes, any myocardial infarction (MI), or target vessel revascularisation (TVR) within 12 months. Nevertheless, the RESOLUTE All Comers study was not powered to show a difference in the incidence of rare events, such as stent thrombosis.
The RESOLUTE International trial was designed to expand the safety data for the new-generation R-ZES, specifically with respect to cardiovascular death and target vessel MI, and to provide clinical outcome data from everyday practice in academic and non-academic coronary intervention centres in 17 countries throughout the world.
Methods
Study design, population, and procedure
The RESOLUTE International trial is a prospective, multicentre, observational registry, in which 2,349 patients with symptomatic coronary artery disease were enrolled at 88 centres in Argentina, Europe, India, and South Africa, between 28 August 2008 and 19 March 2009. Inclusion criteria were broad in order to include an unselected cohort of patients treated in everyday practice. There were no restrictions on clinical indication (stable angina vs. acute coronary syndromes), number of treated vessels and lesions, lesion type, or lesion length. Patients were eligible for enrolment into the study if they had provided written informed consent or signed a patient data release form, and if it was the intention to implant ≥1 new-generation R-ZES. Exclusion criteria were pregnancy, unwillingness to adhere to follow-up requirements, and concurrent participation in another trial that could affect the study procedures.
Patients were to be treated with ≥1 new-generation R-ZES, which elutes zotarolimus from a biocompatible combination of polymers over six months; the design of this stent has been described previously.2 Patients were treated according to routine practices in the local hospitals, including the stent implantation procedure. Although a mixture of stent types was discouraged, the use of non-study stents was left to the operator’s discretion.
Recommended use of dual antiplatelet therapy (DAPT) included aspirin 75 mg for three days prior to the procedure or a periprocedural loading dose of at least 250 mg; and clopidogrel 75 mg for three days prior to the procedure or a periprocedural loading dose of at least 300 mg. The post-procedure regimen included 75 mg aspirin daily indefinitely and clopidogrel 75 mg daily for at least six months. Continuation of DAPT beyond six months was at the treating clinician’s discretion.
Lesion characteristics for the index procedure were determined by visual estimation, and angiographic follow-up was not required. If available, all angiograms related to an event were sent to the independent Clinical Events Committee (CEC; coordinated by Cardiovascular Research Foundation, New York, NY, USA) as part of the adjudication process. Cardiac enzymes, including creatine kinase (CK), its myocardial band isoenzyme (CK-MB), and troponin, were measured post-procedure according to the implanting centre’s standard procedures. If the total CK concentration was within normal limits, CK-MB did not need to be measured, unless guided by local hospital procedures. For all MI events, peak CK and CK-MB values were collected.
Clinical follow-up, either by telephone or in clinic, was carried out at 30 days, six months, and one year.
The study was conducted in accordance with the Declaration of Helsinki, and local ethics committees, if required, approved the study. Alternatively, investigators signed a statement before patient enrolment confirming that no ethics committee approval was required and that this approach was in accordance with local regulations.
Definitions
The primary endpoint was a composite of cardiac death and target vessel Q-wave or non-Q-wave MI at one year. The main secondary endpoint was definite and probable stent thrombosis, according to the Academic Research Consortium (ARC) definition,3 at one year.
All MI data were reported based on extended historical definitions.4 The extended historical definition was developed to better accommodate unselected populations by considering initial clinical setting in various scenarios, and is consistent across the entire RESOLUTE Global Clinical Program, in order to harmonise the clinical event adjudications. For the same reason, the definition of the primary endpoint was based on the historical definition of MI. Any MIs not clearly attributable to a non-target vessel were counted as target vessel MI.
The CEC adjudicated the following events: death (cardiac, vascular, and non-cardiovascular), MI (Q-wave MI and non-Q-wave MI), revascularisation (all target lesion revascularisations [TLRs] and TVRs, with the assessment of clinically driven versus non-clinically driven), and stent thrombosis (according to historical and ARC definitions).
As part of the harmonisation of the CEC responsible for adjudication of events in the various trials in the RESOLUTE Global Clinical Program, 100 primary endpoint events were sent to Harvard Clinical Research Institute (Boston, MA, USA) for cross-adjudication. Any inconsistent adjudication result was discussed at the Global Oversight Committee, which comprised three parties (Cardiovascular Research Foundation [New York, NY, USA], Harvard Clinical Research Institute, and Cardialysis [Rotterdam, The Netherlands]) until consensus was reached. The CEC-Global Oversight Committee was organised to ensure consistency in clinical data review and to harmonise the interpretation of event definitions across the CECs of the RESOLUTE studies.
Additional secondary composite endpoints that were assessed at 30 days, six months, and one year included: death from any cause; target lesion failure, defined as death from cardiac causes, any MI, or clinically driven TLR; major adverse cardiac events, defined as death, MI (Q-wave or non-Q-wave), emergent coronary artery bypass surgery, or repeat clinically driven target lesion percutaneous or surgical revascularisation;3 target vessel failure, defined as death from cardiac causes, any MI (not clearly attributable to a non-target vessel), or clinically driven TVR; and a patient-oriented composite endpoint, defined as death from any cause, any MI (Q-wave and non-Q-wave), or any revascularisation.5
Device success was defined as the attainment of <50% residual stenosis of the target lesion using only the R-ZES. If a non-study stent or no stent was placed because of device failure of the R-ZES, subsequent events were counted as endpoints according to the intent-to-treat principle.
As in the RESOLUTE All Comers trial,1 complex use of the study stent was defined by the following criteria, which were considered off-label when the study was designed: placement of a stent in a patient with ≥1 of the following clinical or lesion characteristics: renal insufficiency, ejection fraction of <30%, occurrence of acute MI within the previous 72 hours, >1 lesion per vessel, ≥2 vessels with stents, a lesion measuring >27 mm in length, bifurcation, bypass grafts, in-stent restenosis, unprotected left main artery, lesions with thrombus, or total occlusion.
Data collection and management
All clinical data were collected prospectively and documented in a web-based case report form. For quality control purposes, study monitors visited all sites, and verified all source data for a random selection of 25% of the enrolled patients. In addition, remote data monitoring was done through an on-line system. An independent data safety and monitoring board provided periodic study review.
Statistical methods
The primary analytical population (intention-to-treat set) consisted of all enrolled patients in whom an R-ZES was attempted and/or implanted. Preliminary results from the E-Five registry suggest a rate of approximately 3% for cardiac death and MI at 12 months in an unselected population.6 With this assumption, a sample size of 2,200 patients will provide a 95% confidence interval with a margin of error of <0.8% for the primary endpoint, which allows for a maximum rate of 5% patients lost to follow-up. Categorical variables are reported using percentages and counts, and continuous variables as means±standard deviations (SDs). Event rates were calculated based on the number of patients with completed follow-up at the respective time point. Fisher’s exact test was used to calculate 95% confidence intervals for binary variables. In addition, cumulative event rates were calculated and graphically described according to the Kaplan-Meier method.
Results
Patient population and follow-up
The RESOLUTE International study comprised 2,349 patients with 3,147 lesions. Table 1 summarises the baseline demographics of these patients. The mean age of these patients was 63.5 years and 77.8% were male. The study comprised the entire clinical spectrum of coronary artery disease. Almost one half (46.0%) of the patients had an acute coronary syndrome; 27.0% presented with MI. Moreover, the study comprised a high proportion of patients with diabetes (30.5%). Most of the lesions were located in the left anterior descending artery, but left main lesions were also treated in 2.6% as well as lesions in bypass grafts in 1.8%. Overall, 57.1% of the lesions were classified as complex (American College of Cardiology/American Heart Association type B2/C), including chronic total occlusions in 6.3% and bifurcation lesions in 18.2% (on a lesion level). Almost one half of the patients had lesions that were ≤2.75 mm in diameter or >18 mm in length (Table 1). In more than two thirds of the patients, stent use met one of the predefined complexity criteria. Table 2 summarises the procedural characteristics. Multiple vessel intervention was performed in 14.0% of patients.
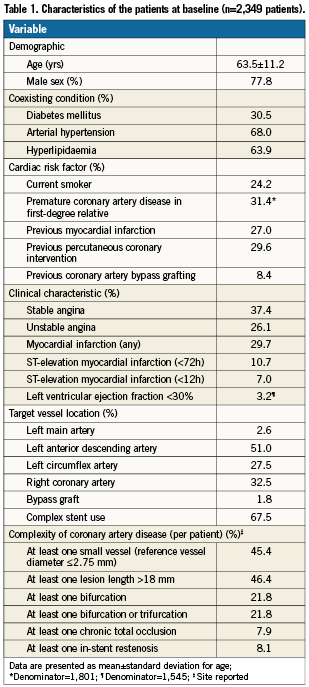
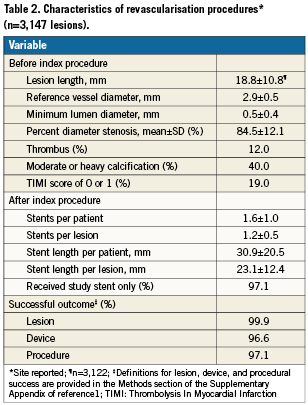
On average, 1.3 lesions were treated with 1.6 stents per patient. Clinical follow-up was complete in 99.8% of the patients at day 30, in 99.4% at six months, and in 97.9% at 12 months. At 30 days, 96.9% of the patients were on DAPT. The proportion was 95.6% at six months and 91.3% at 12 months.
Clinical outcomes
Table 3 and Figure 1 summarise clinical outcomes. The composite cumulative incidence of cardiac death and target vessel MI, the primary endpoint of the study, was reached in 4.3% (95% CI: 3.5% to 5.2%) of patients (Table 3). As shown in Figure 1, most of the events occurred early, with an incidence of the primary endpoint of 2.9% at 30 days and of 1.3% from day 31 to 1-year follow-up. The incidence of cardiac death was 1.4%. Thus, target vessel MI constituted the majority of primary endpoint events and reached a cumulative incidence of 3.1% at 1-year follow-up (Table 3); 37 (1.6%) of the 71 target vessel MI occurred periprocedurally.
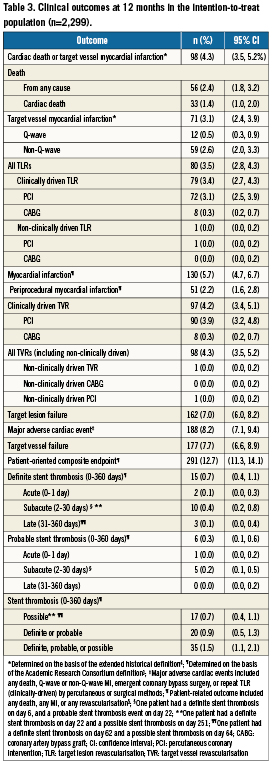
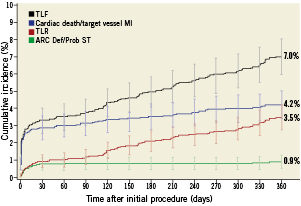
Figure 1. Kaplan–Meier estimates for cumulative incidence with 95% confidence intervals for the primary endpoint (cardiac death and target vessel myocardial infarction), definite and probable stent thrombosis, target lesion revascularisation, and target lesion failure. ARC Def/Prob ST: Academic Research Consortium definite/probable stent thrombosis; MI: myocardial infarction; TLF: target lesion failure; TLR: target lesion revascularisation
At one year, the cumulative composite incidence of ARC definite and probable stent thrombosis, the main secondary endpoint of the study, was 0.9% (Figure 1, Table 3). Similar to the primary endpoint, most definite and probable stent thromboses occurred within the first 30 days (0.7%), whereas the incidence of definite and probable stent thrombosis from day 31 to the end of follow-up was 0.1% (Figure 1). During 1-year follow-up, TLR was needed in 3.4% of the patients (Figure 1, Table 3). The 1-year incidences of target lesion failure (TLF) and major adverse cardiac events were 7.0% and 8.2%, respectively (Table 3).
As shown in Figure 2, the incidences of the primary endpoint as well as TLR were consistent throughout various lesion subsets and clinical characteristics. There was a trend towards higher incidences of the primary endpoint in diabetic patients (5.3% vs. 3.8% in non-diabetic patients, p=0.12).
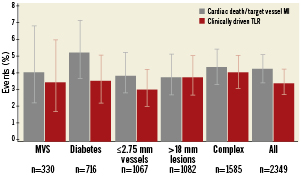
Figure 2. Frequency of the primary endpoint (cardiac death and target vessel myocardial infarction) and target lesion revascularisation in various lesion and patient subsets. MI: myocardial infarction; MVS: multivessel stenting; TLR: target lesion revascularisation
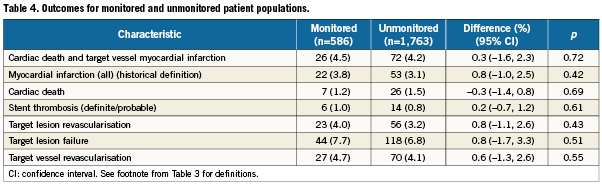
Per protocol, 586 randomly selected patients were monitored. Almost all of the baseline characteristics of monitored and unmonitored patients were not significantly different (data not shown). Exceptions were a higher frequency of MI >72 hours, ST elevation <12 hours, and left main vessel location, and a lower frequency of stable angina and side-branch stenting only, among monitored patients. As shown in Table 4, outcomes for monitored and unmonitored patients did not differ significantly, with point estimates for the differences ranging from 0.3% for cardiac death to 0.8% for target vessel failure.
Discussion
The RESOLUTE International Registry yields additional safety data for the new-generation R-ZES. Despite complex use of the R-ZES in 67% of the patients and a prevalence of diabetes mellitus of 30%, cardiovascular death and target vessel MI, our primary endpoint, were noted in only 4.3% of the patients at one year. Moreover, the 1-year incidence of ARC definite and probable stent thrombosis was 0.9%. Importantly, most of these events occurred soon after intervention, suggesting that at least some were procedure-related. Overall in our study, target lesion reintervention rates were low, which further confirms the efficacy of the R-ZES in minimising neointima formation. Our study results need to be considered in face of the lesion complexity in this study, with 57% type B2/C lesions, 46% of patients with lesions >18 mm, 45% of patients with vessel sizes ≤2.75 mm, and 22% of patients with bifurcation lesions.
The RESOLUTE International Registry corroborates the main results of the randomised RESOLUTE All Comers trial.1 With respect to target lesion failure (TLF), the RESOLUTE All Comers trial demonstrated non-inferiority of the new-generation R-ZES compared with an everolimus-eluting stent. In the current study, TLF was numerically even lower than in the randomised trial (7.0% vs. 8.2%). Consistent results were also obtained for TVF (7.7% vs. 9.0%), clinically driven TLR (3.4% vs. 3.9%), and clinically driven TVR (4.2% vs. 4.9%). Such favourable outcomes in our study may not be attributed to a lower risk profile. Compared with RESOLUTE All Comers, the prevalence of diabetes was substantially higher in RESOLUTE International (30% vs. 24%), as were the proportions of patients with long lesions (46% vs. 18%). Most of the other baseline variables were indicative of a similar risk profile in the registry as compared with the randomised trial, except for lower prevalence of small vessel lesions (45% vs. 68%) and chronic total occlusions (8% vs. 16% patients having at least one lesion with TIMI 0 flow). Thus, taken together, the RESOLUTE randomised trial and the registry establish a solid database for the efficacy of the R-ZES.
A finding in the RESOLUTE All Comers study was a higher incidence of definite and probable stent thrombosis of the R-ZES (p=0.05) compared with the everolimus-eluting stent.1 This finding was difficult to interpret, as RESOLUTE All Comers was not powered to address this rare event. Moreover, there was no significant difference with respect to any stent thrombosis (by the ARC definition) or to other safety endpoints. Nevertheless, the authors of the RESOLUTE All Comers report requested further investigation of this issue in larger populations of patients. To this end, it is reassuring that the observed 1-year incidence of definite and probable stent thrombosis in RESOLUTE International was lower than that in the RESOLUTE All Comers study (0.9% vs. 1.6%) and similar to that of the control stent (0.7%) in RESOLUTE All Comers.
RESOLUTE International comprised the entire spectrum of PCI procedures. The efficacy outcome of the R-ZES was comparable across all lesion and patient subsets analysed. Neither did we identify a safety problem in any of these subsets. Only diabetes mellitus appeared to be associated with an increased risk of cardiovascular death and target vessel MI. Yet, the difference between diabetic and non-diabetic patients was smaller than what might have been anticipated from previous studies.7
Among the various lesion and patient subsets, the complex patient population deserves particular attention. In RESOLUTE All Comers, complex patients experienced a higher incidence of cardiac death and target vessel MI than non-complex patients, irrespective of whether the R-ZES or the everolimus-eluting stent was placed.8 Both stent types proved to be safe and effective, regardless of complexity. With the same entry criteria and the same definition for complexity as in RESOLUTE All Comers, the complex patient population of RESOLUTE International demonstrated a similar 1-year incidence of cardiac death and target vessel myocardial infarction myocardial (4.4%) as the complex patient population in RESOLUTE All Comers (3.8%).8 This observation strengthens the inference from RESOLUTE All Comers that the R-ZES is equally well suited for complex and non-complex PCI.
Limitations
Missing post-interventional cardiac enzyme measurements in 46% of the patients will have resulted in some underestimation of periprocedural MIs. Nonetheless, periprocedural MI was only a minor contributor to the 1-year cumulative incidence of this event, which mitigates the impact of missing post-interventional cardiac enzyme measurements.
In addition, we need to consider underreporting in the 75% unmonitored patients. With the exception of cardiac death, all observed incidences for relevant outcome events were lower in unmonitored patients than in monitored. Differences in event rates between monitored and unmonitored patients were, however, numerically small and did not reach statistical significance. Hence, although some degree of underreporting needs to be acknowledged, its impact on the overall outcomes may be considered too small to affect the principal message of RESOLUTE International.
As the study was designed to confirm the safety and effectiveness of the R-ZES with respect to clinical endpoints, angiographic endpoints were beyond our scope. Clinical endpoints could be adequately adjudicated by the CEC independent of an angiographic core laboratory. This is also true for the total number of TVR, which we report. Core laboratory analysis may interfere, however, with the proportion of TVRs adjudicated as TLRs and with the proportion of TVR adjudicated as clinically driven. Yet, as there was no study-mandated angiographic follow-up, the proportion of on non-clinically driven TLR is not a major source of concern.
The current report is limited to the 1-year outcomes of RESOLUTE International. Pre-specified analyses of 2-year outcomes will yield more long-term data on the safety and efficacy of the R-ZES.
Implications
In the past, new generation stent designs did not always advance safety and efficacy.9 Therefore, to qualify for routine clinical use, modifications in stent design need to be tested in adequately designed studies with sufficient numbers of patients included.10 With respect to the new-generation R-ZES, RESOLUTE International, which we present here, is a further step in this endeavour. With 2,349 patients included, RESOLUTE International yields safety data with narrow CIs. Moreover, RESOLUTE International supports the generalisability of the RESOLUTE All Comers study results. The randomised RESOLUTE All Comers study demonstrated the efficacy of the R-ZES by showing non-inferiority to the comparator everolimus-eluting stent with respect to target lesion failure. RESOLUTE International shows consistent results for the R-ZES in a wide range of lesion subsets, comorbid conditions and clinical settings.
Acknowledgements
Sophie Rushton-Smith, PhD, provided editorial support, limited to editing, checking content and language, formatting, referencing, and preparing tables and figures, but without editing of the scientific content.
Funding
The RESOLUTE International Registry is funded by the Medtronic Bakken Research Center, Medtronic CardioVascular, Maastricht, The Netherlands.
Appendix
Participating centres and investigators
Austria
Donauspital im SMZ-Ost: H. Weber; Universitätsklinik Innsbruck: N. Moes; Landeskrankenhaus Graz-West: H. W. Schuchlenz
Belgium
Heilig Hart Ziekenhuis: F. Stammen; Virga Jesseziekenhuis: E. Benit; CHU Sart Tilman: V. Legrand; Hôpital Erasme: E. Stoupel; CHU de Charleroi: J. Lalmand; Clinique St Luc: E. El-Khoury; Universitair Ziekenhuis Antwerpen: C. Vrints
Finland
Helsinki University Hospital: M. Laine
Germany
Herz-Zentrum Bad Krozingen: F.-J. Neumann; Schwarzwald-Baar Klinikum Villingen-Schwenningen: W. Jung; Ambulantes Herzzentrum Kassel: K.-F. Appel; Krankenhaus Landshut-Achdorf: J. Dietl; Klinikum Leverkusen: P. Schwimmbeck; Schüchtermann-Klinik: N. Franz; Evangelisches Krankenhaus Düsseldorf (EVK): E. Vester; Klinikum Oldenburg gGmbH: A. Elsässer; Marienhospital: T. Wichter; Universitätsklinikum Rostock: C. Nienaber; Klinikum Esslingen: M. Leschke; CardioVasculäres Centrum Frankfurt Sankt Katharinen: H. Sievert; St.-Marien-Hospital Lünen: C. Perings; Krankenhaus Bethanien für die Grafschaft Moers: H.-J. Mertens; Krankenhaus St. Franziskus: J. vom Dahl; Diakonissen Speyer-Mannheim: H.Schwacke; Vivantes Klinikum Auguste Viktoria: H. Schühlen
The Netherlands
UMC St Radboud: H. Gehlmann; Alysis Zorggroep: M. Tjon Joe Gin; UMC Utrecht: P. Stella; Academisch Medisch Centrum: J.P. Henriques; Medisch Centrum Alkmaar: R. Hautvast; Academisch Ziekenhuis Maastricht: J. Waltenberger
Norway
Stavanger Universitetssykehus: D. Nilsen; Sörlandet Sykehus Arendal: M. Uchto
Portugal
Hospital de Santa Cruz: P. Gonçalves; Hospital Santa Marta: L. Patricio; Serviço de Saúde da Região Autónoma da Madeira, E.P.E.: J. Araújo
Spain
Hospital Gregorio Marañon: J Soriano; Hospital de San Juan de Alicante: R. F. López; Hospital Universitario Infanta Cristina: J. R. López; Hospital Germans Trias i Pujol: J. Mauri; Hospital del Mar: A. Serra; Hospital San Pedro de Alcántara: J. Fernández; Hospital General Universitario de Guadalajara: J. Balaguer; Hospital de Jaen: M. F. Guzman; Hospital 12 de Octubre: F. Hernández; Hospital Carlos Haya: C. Urbano; Hospital Virgen de la Macarena: R. Ruiz; Hospital Joan XXIII: L. Kristicevic; Hospital Universitario Nuestra Señora de Candelaria: H. Pérez; Hospital General Yagüe: J. M. Durán; Hospital Universitario Virgen de la Arrixaca: M. Valdés; Hospital General de Asturias: I. Lozano; Hospital Universitario de Salamanca: I. Santos; Hospital Universitario Virgen de la Victoria: J. M. Hernández
Switzerland
Stadtspital Triemli: F. Eberli; Hôpital Fribourgeois - Hôpital cantonal: S. Cook; Hôpital de la Tour: E. De Benedetti; Clinique Cecil: J.-J. Goy; Kantonsspital St.Gallen: H. Rickli; Etablissement hospitalier de Sion: G. Girod
United Kingdom
St Thomas` Hospital: S. Redwood; Craigavon Area Hospital: I. Menown; Royal Free Hospital: R. Rakhit; Dorset General Hospital: F. Witherow; Glenfield Hospital: G. Richardson; Hairmyres Hospital: B. O`Rourke; Hull Royal Infirmary: F. Alamgir
Czech Republic
Faculty Hospital of Kralovske Vinohrady: T. Budešínský
Estonia
Tartu University Hospital: T. Hermlin
Greece
Errikos Dunant: V. Tzifos; Athens Medical Center: G. Papaioannou; Agios Loukas: D. Tsikaderis; HYGEIA: A. Dimas
Slovakia
OIK NÚSCH: V. Fridrich
Argentina
Instituto Cardiovascular de Buenos Aires (ICBA): J. A. Belardi; Sanatorio Allende: H. Londero
India
The Heart Care Clinic: M. Chag; Bombay Hospital & Medical Research Centre: B. Goyal; All India Institute of Medical Sciences: V. Kumar Bahl; Madras Medical Mission - Institute of Cardiovascular Diseases: A. Mullasari Sankardas; B. M. Birla Heart Research Centre: A. Mishra
South Africa
Unitas Hospital: C. Badenhorst; Panorama Medi-Clinic: J.P. Roux; Milpark Hospital: G. Cassel; Vincent Pallotti Hospital: A. Horak
Conflict of interest statement
F.J. Neumann has nothing to disclose. P. Widimsky receives occasional speaker’s honoraria from Medtronic. J.A. Belardi serves on an advisory board to Medtronic CardioVascular.

