Abstract
Aims: We conducted a pooled post hoc analysis (RESOLUTE All Comers and RESOLUTE International) of patients who had the Resolute® zotarolimus-eluting stent (R-ZES) implanted in revascularised total occlusions (TO) compared with patients treated with R-ZES for non-occluded lesions
Methods and results: Patients were divided into three groups: chronic TO (CTO; n=256), non-chronic TO (n=292), and no occlusion (n=2,941). Clinical and safety outcomes assessed through two years included target lesion failure (TLF: cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularisation) and Academic Research Consortium definite or probable stent thrombosis. The rate of TLF at two years was not significantly different among patients in the CTO (9.1%), TO (9.8%), and no occlusion (10.4%) groups (log-rank p=0.800); neither were the components of TLF. Definite or probable stent thrombosis occurred more frequently in the TO group (2.8% vs. 1.2% in the CTO and 1.1% in the group with no occlusion, p=0.027). There were 10 late and six very late stent thrombosis events.
Conclusions: Apart from a higher rate of stent thrombosis in patients with TO, patients with totally occluded coronary arteries who receive revascularisation with an R-ZES have clinical outcomes comparable to those who receive a similar stent in non-occluded lesions.
Introduction
Total coronary occlusions represent a specific challenge in invasive cardiology. Whereas a culprit coronary occlusion associated with acute coronary syndrome should be treated immediately or with limited time delay by mechanical revascularisation according to current guidelines, invasive treatment of subacute coronary occlusions remains controversial1,2. The value of revascularisation of chronically totally occluded lesions is also a matter of debate3, although recent research indicates that the presence of a totally occluded artery is an independent predictor of mortality in patients with multivessel disease4 and that full revascularisation improves survival5.
The procedural and clinical results of percutaneous coronary intervention (PCI) for occluded coronary arteries rely on successful wiring technique, pushability and trackability of the balloon and the stent, and stent conformability. An antiproliferative drug released from the stent has also proved to be important to limit the risk of in-stent restenosis and the need for revascularisation6,7.
Whereas original proof of safety and efficacy of the first-generation drug-eluting stents (DES) was based on trials that included only patients with simple coronary lesions, subsequent randomised comparisons with bare metal stents (BMS) supported the initial findings that DES efficacy was at least as pronounced in patients with complex lesions including total coronary occlusions8,9. Large-scale randomised trials and meta-analyses focusing on the long-term outcome of patients treated with PCI and first-generation DES compared with BMS for acute myocardial infarction (MI) have relatively consistently found a reduced need for reintervention, although findings with regard to the occurrence of late stent thrombosis have not been consistent10-12.
Recently, the Resolute® zotarolimus-eluting stent (R-ZES) (Medtronic CardioVascular, Santa Rosa, CA, USA) and the everolimus-eluting stent were evaluated in a large all-comers trial with virtually no restrictions on inclusion13, and concomitantly the safety and efficacy have been tested in two international registries14-16. The R-ZES was found to be non-inferior to the everolimus-eluting stent13 and safe and effective in single-arm registries14-16. The purpose of this study was to evaluate the safety and efficacy of the R-ZES in a subset of patients with totally occluded coronary arteries included in both the randomised trial and the largest registry.
Methods
STUDY POPULATION
The present data are derived from the RESOLUTE All Comers (ClinicalTrials.gov, NCT00617084) and the RESOLUTE International trial (ClinicalTrials.gov, NCT00752128) (Figure 1). The RESOLUTE All Comers trial is a multicentre, randomised trial comparing the results after implantation of the R-ZES with the everolimus-eluting stent for coronary artery disease in 2,292 patients with no restrictions to inclusion other than intolerance to a study drug, metal alloys, or contrast media, pregnancy, planned surgery within six months after the index procedure, and participation in another trial13. The RESOLUTE International trial is a multicentre, prospective, observational registry enrolling 2,349 patients after implantation of at least one R-ZES for coronary artery disease with the same restrictions as above except for planned surgery16. The reference diameter of the treated vessel should adhere to the available stent balloon sizes between 2.25 and 4.0 mm.
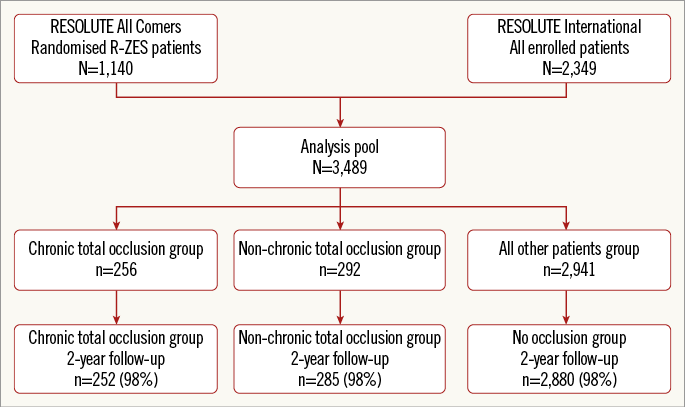
Figure 1. Flow chart of patient inclusion. R-ZES: Resolute zotarolimus-eluting stent
After detailed information, all patients consented in writing to participate in both the trial and the registry. Local ethics committees approved the protocols, and both studies were conducted in accordance with the Declaration of Helsinki.
PROCEDURES AND DEFINITIONS
The definition of a total coronary occlusion was completely interrupted contrast filling (Thrombolysis In Myocardial Infarction [TIMI] flow 0) in the coronary artery. We divided these patients into those with a non-chronic total occlusion (TO) of <3 months’ duration, and those with an estimated duration of ≥3 months (CTO).
PCI was performed according to local standards, and post-procedure medical regimens included acetylsalicylic acid and clopidogrel 75 mg daily for at least six months. Clinical follow-up was performed after two years.
Apart from procedural data, the following outcomes were collected at two-year follow-up: target lesion failure (TLF) defined as cardiac death, target vessel myocardial infarction (TVMI), and target lesion revascularisation (TLR); target vessel failure defined as cardiac death, TVMI, or target vessel revascularisation (TVR); major adverse cardiac events (MACE) defined as death, MI, TLR, or emergency coronary artery bypass graft surgery. In addition, the stent thrombosis rate was recorded according to the Academic Research Consortium definitions17.
All deaths were considered cardiac unless an undisputed non-cardiac cause was documented. MI was defined according to extended historical definitions in order to harmonise the clinical event adjudications18.
Lesion success was defined as <50% residual stenosis of the target lesion after stent implantation, device success as lesion success using the allocated stent, and procedure success as lesion success and no in-hospital MACE.
Statistical analysis
For this analysis all patients from RESOLUTE International (n=2,349) and R-ZES patients from the RESOLUTE All Comers trial (n=1,140) were pooled. The pooled cohort was divided into three groups: patients with CTO (n=256), non-chronic TO (n=292), and no occlusion (n=2,941).
Categorical variables are reported as percentages and counts. Differences between groups were analysed using Fisher’s exact test. Continuous variables are presented by their means and standard deviations. The Kaplan-Meier method was used to create survival estimates of the time-to-event variables, and the log-rank test to test differences in the estimates; p-values <0.05 were considered significant.
Results
Of the 2,292 patients enrolled in the RESOLUTE All Comers trial, 1,140 had an R-ZES implanted. In addition, 2,349 patients were enrolled in the RESOLUTE International trial. Thus, in total 3,489 patients had an R-ZES implanted. Of these, 256 patients (7.3%) were treated for a CTO, 292 (8.4%) for a TO (86% with acute coronary syndrome), and 2,941 (84.3%) for a non-occluded lesion. Two-year clinical follow-up was available for 98% of patients in each group (Figure 1).
BASELINE CHARACTERISTICS
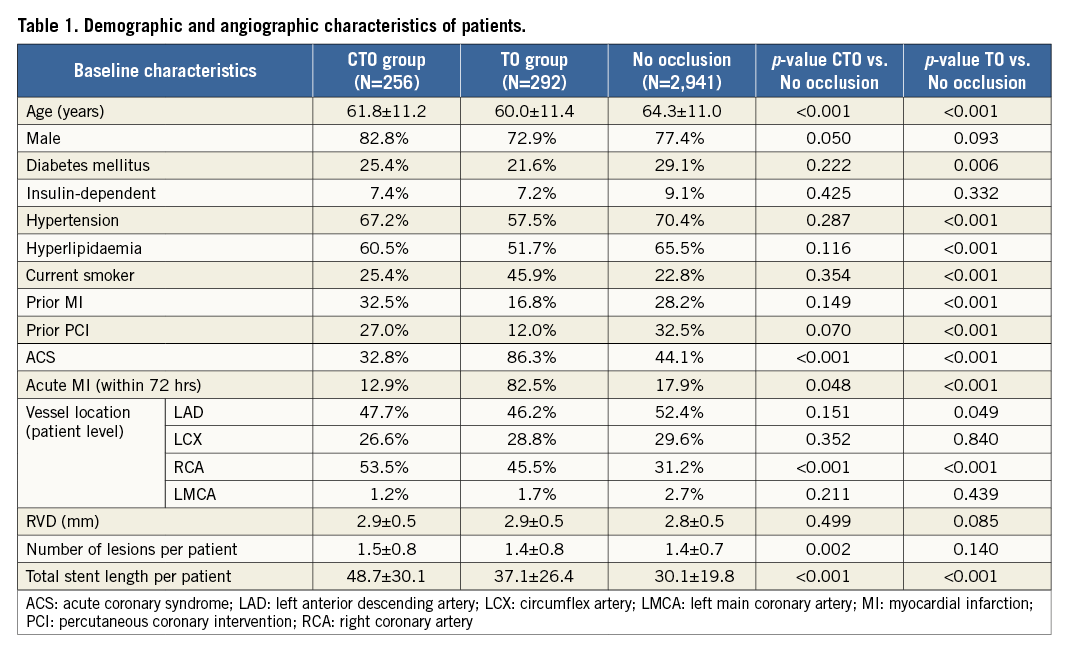
Demographic and procedural data are presented in Table 1. Compared to patients without, those with a total occlusion were younger, more often had a lesion in the RCA, and had a longer total stent length (longest for the CTO group). There was a lower proportion of patients with diabetes, hypertension, hyperlipidaemia, previous MI or PCI but more smokers in the TO group.
CLINICAL OUTCOMES
Lesion and procedure success were slightly lower in the CTO group, whereas safety outcomes were similar among all patient groups, except for a higher rate of stent thrombosis in TO patients (Table 2). Despite the higher rate of stent thrombosis in this group, the cumulative incidence of TLF was similar: 9.1% in CTO patients vs. 9.8% in TO patients vs. 10.4% in patients with non-occluded lesions, log-rank p=0.80 (Table 2 and Figure 2A). No differences were found in all-cause mortality, cardiac death, TVMI or TLR (Figure 2B and Figure 2C).
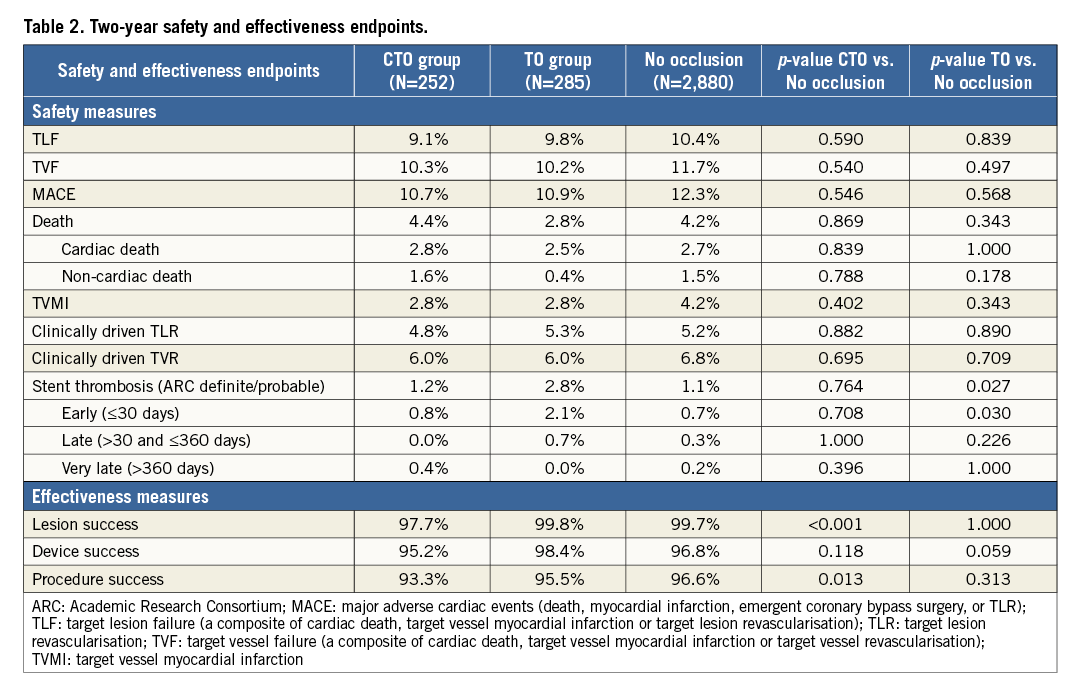
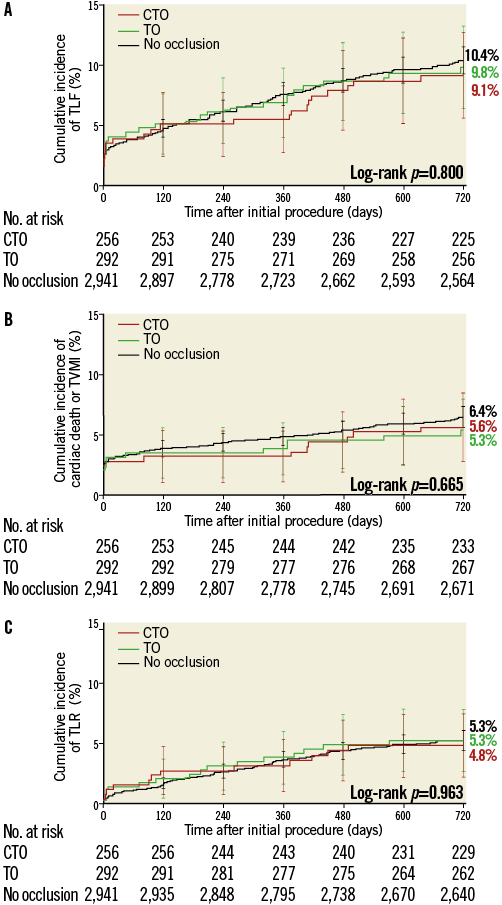
Figure 2. Target lesion failure and components stratified by analysis group. Kaplan-Meier cumulative incidence of target lesion failure (TLF) (A), cardiac death or target vessel myocardial infarction (TVMI) (B), and clinically driven target lesion revascularisation (TLR) (C) in the chronic total occlusion (CTO) group (n=256), the non-chronic total occlusion (TO) group (n=292) and patients without occlusions (n=2,941).
Only 10 cases of late and six cases of very late definite or probable stent thrombosis occurred in the three groups (Table 2). Stent thrombosis events occurred on and off dual antiplatelet therapy (DAPT) as follows by group: two on and one off DAPT in the CTO group, seven on and one off DAPT in the TO group, and 26 on and four off DAPT in all others. (Three patients’ DAPT status at the time of the event could not be ascertained).
Discussion
That stent thrombosis was more frequent in patients who had an R-ZES implanted in a TO in our analysis is probably related to recent MI in the territory of the target lesion in some of these patients. This explanation is supported by the higher proportion of acute coronary syndromes and acute MI in the TO group, which have been identified as significant risk factors of stent thrombosis19. Additionally, despite the higher incidence of stent thrombosis in the TO group in our analysis, it is reassuring that patients in the TO group, including those with an acute MI, had a similar incidence of TLF as compared to both patients in the CTO group and those with no occlusion.
Previous studies have established the safety and efficacy of DES compared with BMS implantation in patients with complex coronary artery disease. In patients with totally occluded coronary arteries, first-generation DES have proved superior in most settings in patients with stable angina pectoris as well as in patients with acute coronary syndromes20-23. However, it should be stressed that, apart from trials evaluating the use of first-generation DES in patients with acute MI, most of the clinical data have been generated from registries6,7, whereas only a few randomised trials have focused on the safety and efficacy of DES compared with BMS treatment of patients with coronary occlusions of longer duration8,9. A recently published randomised study24 compared a first-generation DES (sirolimus-eluting stent) to both generations of ZES (Resolute and Endeavor®; Medtronic CardioVascular) in total coronary occlusions, and found angiography at eight months and clinical outcomes through 12 months between R-ZES and its comparator were similar. Long-term outcomes have not yet been reported24.
The present study describes the clinical outcome after implantation of current-generation DES in total coronary occlusions in a variety of clinical settings. Even though acute coronary syndromes, and in particular ST-segment elevation MI, represent clinical entities that were recently regarded “off-label” indications for DES implantation, our findings suggest that R-ZES is both safe and effective in patients suffering from these diseases. The one-year clinical outcome of patients enrolled in the RESOLUTE International trial showed consistent efficacy results compared with the randomised RESOLUTE All Comers trial16. In addition, in comparison with the randomised trial, a lower level of definite and probable stent thrombosis was reported in the registry. Similar favourable clinical outcomes have been reported from a corresponding registry conducted in a US population15. Extremely low rates of definite and probable stent thrombosis were found in that study and only in stents with a diameter of 2.25 mm.
The clinical outcome is quite similar for patients who have had an R-ZES or an everolimus-eluting stent implanted in a variety of lesion types and clinical situations25. In addition, a 13-month angiographic subgroup analysis of the all-comers trial demonstrated that, even in patients with recent MI, heart or renal failure or with complex anatomy of the culprit lesion (including total coronary occlusions), the level of binary in-stent restenosis was only approximately 5% in both stent groups26. In that trial the rate of stent thrombosis was extremely low in both groups, even in complex patients who had a higher occurrence of TLF. Also, a pooled analysis of the same all-comers trials we analysed showed no difference in cardiac death or target vessel myocardial infarction between R-ZES patients with or without in-stent restenosis27. R-ZES appeared to be equally effective at treating BMS and DES in-stent restenosis.
In consecutive cohorts of patients treated with PCI, CTOs represent approximately 10% of the lesions28, and the procedural success rate seems to be dependent on the anatomical characteristics of the lesion rather than the estimated duration of the occlusion29. With regard to the clinical utility of recanalisation of a CTO we have to await the results of ongoing large randomised trials. Until they are available we have to rely on the reports of registries. The results of such a registry indicated a lower mortality in successfully recanalised CTOs especially in the LAD and Cx territories30. The findings of the present study suggest that patients treated with the R-ZES in sizes from 2.25 to 4.0 mm in diameter after recanalisation of totally occluded coronary arteries have the same favourable outcome after two years compared with patients treated for non-occlusive disease, despite the fact that adequate treatment of a CTO requires considerably longer segments of stents to cover the lesions.
Despite the occurrence of a higher rate of stent thrombosis in this group, it is reassuring that patients in our TO group, including those with an acute MI, have similar levels of TLF compared with both the CTO group and the patients without an occluded lesion. Thus, a high level of safety, indicated by a low rate of cardiac mortality, MI, and revascularisation two years after treatment with the R-ZES for a totally occluded coronary artery in a wide spectrum of clinical conditions in both the randomised trial and the registry, indicates that current-generation DES should be used with limited concern for long-term adverse events. Virtually superimposed two-year curves of the cumulative incidence of TLF and its components in patients treated with R-ZES (whether the lesion was a total occlusion or not) support this concept (Figure 2).
Study limitations
Limitations of the study are related to combined observations from a subgroup analysis of a randomised trial added to observations of a registry in order to focus upon the clinical outcome of patients. In the RESOLUTE All Comers, close monitoring revealed that nearly 50% of all eligible patients were actually enrolled in the randomised comparison of the R-ZES and an everolimus-eluting stent13. Similar monitoring was not performed in the registry, adding a certain risk of selection bias in that part of the study. Thus, the results should be interpreted accordingly. Despite differences in demographic data, which reflect differences in their clinical status, patients with CTO, TO, and non-occluded lesions had similar outcomes. Finally, the number of patients within each analysis group does not permit an assessment of DAPT effects on stent thrombosis, an important topic31,32 that has been analysed in the Global RESOLUTE clinical programme as a whole33. The stent thrombosis events observed were rare and do not allow evaluation of the many factors that should be considered in an analysis of DAPT on stent thrombosis, including timing of interruption, duration of interruption, and which component (aspirin or thienopyridine) was interrupted/discontinued. Nonetheless, the finding of fewer stent thromboses in patients off DAPT is concordant with a previous report33.
Conclusions
This study supports previous findings concerning the safety and efficacy of current-generation DES not only in patients with simple but also in those with complex coronary lesions. Our study suggests that patients treated with R-ZES after recanalisation of totally occluded coronary lesions have a clinical outcome that is at least as favourable as those with non-occlusive disease.
| Impact on daily practice The original term “off-label indication” for treatment of certain coronary artery lesions with stent implantation referred to the fact that these lesions had usually been excluded from previous randomised trials, thus lacking the evidence of a favourable outcome of the treatment. Totally occluded lesions, be they due to acute coronary syndromes or chronic disease, belonged to this category. Recent trials, including those reported in the present document, have shown that implantation of new-generation drug- (in casu zotarolimus) eluting stents is both efficient and safe in re-opened totally occluded coronary artery lesions, despite a wide spectrum of origin. |
Acknowledgements
We thank C. Gilbert and T. Peoples for editorial assistance and M. Liu and Y. Peng for statistical analysis oversight.
Funding
The studies represented in this analysis were sponsored by Medtronic, Inc.
Conflict of interest statement
H. Kelbæk and P. Stella have received research grant support via institutional grants from Medtronic, Inc. S. Windecker has received research grant support via institutional grants from Abbott, Biotronik, Biosensors, Boston Scientific, Cordis, Medtronic, and St. Jude Medical. P. Widimský has received occasional speaking honoraria from Medtronic. J.A. Belardi has served as a consultant and as a speaker for Medtronic and Eli Lilly. S. Silber has received grant, travel, and analysis support from Medtronic for the RESOLUTE All Comers trial. L. Holmvang, P.E. Buszman, F.J. Neumann, F.R. Eberli, G. Richardt, and P.W. Serruys have no conflicts of interest to declare.

