Abstract
Aims: To assess three-year clinical outcome following randomised use of the second-generation Resolute zotarolimus-eluting stent (ZES) and the XIENCE V everolimus-eluting stent (EES). For Resolute ZES and randomised use, outcome data ≥3 years are relatively scarce.
Methods and results: The TWENTE trial examined 1,391 patients with stable angina or non-ST-elevation acute coronary syndromes, of whom 21.6% were diabetics, 70.1% had complex B2 or C lesions and 77.4% had “off-label” indications for DES use. Three-year follow-up data were obtained in 1,381 patients (99.3%; 10 withdrawals). Adverse clinical events were independently adjudicated. The primary endpoint target vessel failure (TVF), a composite of cardiac death, target vessel-related myocardial infarction and clinically indicated target vessel revascularisation, was 12.1% for Resolute ZES and 13.4% for XIENCE V EES (p=0.50). Cardiac death rates were 1.9% vs. 3.5% (p=0.06); the other individual components of TVF also showed no significant between-group differences. The rates of definite-or-probable stent thrombosis (1.4% vs. 1.6%, p=0.82) and very late stent thrombosis (0.6% vs. 0.4%, p=1.0) did not differ between the groups.
Conclusions: Three-year follow-up data of patients included in the randomised TWENTE trial demonstrated similar and sustained safety and efficacy of Resolute ZES and XIENCE V EES.
Introduction
Clinical follow-up data beyond two years provide valuable information on the long-term safety of drug-eluting stents (DES). The Resolute zotarolimus-eluting stent (ZES) (Medtronic Inc., Santa Rosa, CA, USA) is a second-generation DES that is widely used, but only a single randomised study, the RESOLUTE All-Comers trial, has published outcome data beyond two years1. The randomised TWENTE trial has previously demonstrated, in a broad study population, the non-inferiority of the Resolute ZES compared to the XIENCE V everolimus-eluting stent (EES) (Abbott Vascular, Santa Clara, CA, USA)2,3. Meanwhile, three-year outcome data of the TWENTE trial have been obtained, which contribute significantly to the knowledge about the longer-term safety and efficacy of second-generation DES.
Methods
Study design, definitions of clinical endpoints, characteristics of patients, lesions, procedures and the one- and two-year clinical outcomes of the investigator-initiated, patient-blinded, randomised TWENTE trial (ClinicalTrials.gov NCT01066650) have been previously reported2,3. The TWENTE trial enrolled 1,391 patients with stable angina or non-ST-elevation acute coronary syndromes, of whom 21.6% were diabetics, 70.1% had complex lesions and 77.4% fulfilled at least one criterion of off-label DES use2. More than 80% of all eligible patients were enrolled in this randomised clinical trial4. Between two- and three-year follow-up, the external CRO Diagram (Zwolle, The Netherlands) monitored clinical outcome in 10% of randomly selected patients and organised the adjudication of adverse events by an independent clinical events committee. The TWENTE trial and follow-up have been approved by the institutional medical ethics committee, complied with the Declaration of Helsinki and patients provided written informed consent. Clinical endpoints were defined according to the Academic Research Consortium (ARC)5; myocardial infarction (MI) was classified according to the extended historical definition6. Primary endpoint of TWENTE was target vessel failure (TVF) at one year, a composite of cardiac death, target vessel-related MI and clinically indicated target vessel revascularisation (TVR). A p-value <0.05 was considered statistically significant and statistics were performed as appropriate and corresponding to previous reports2,3 using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
We obtained three-year follow-up in 1,381 patients (99.3%; i.e., all enrolled patients except 10 withdrawals). Both the Resolute ZES and the XIENCE V EES groups showed a favourable outcome with a similar incidence of TVF (84/692 [12.1%] vs. 92/689 [13.4%], plog-rank=0.58; Figure 1, Table 1), a composite of cardiac death, target vessel-related MI and clinically indicated TVR. Between DES groups, there was also no significant difference in a patient-oriented composite endpoint, consisting of all-cause death, any MI or any revascularisation (120/692 [17.2%] vs. 114/689 [16.5%], p=0.69; Table 1).
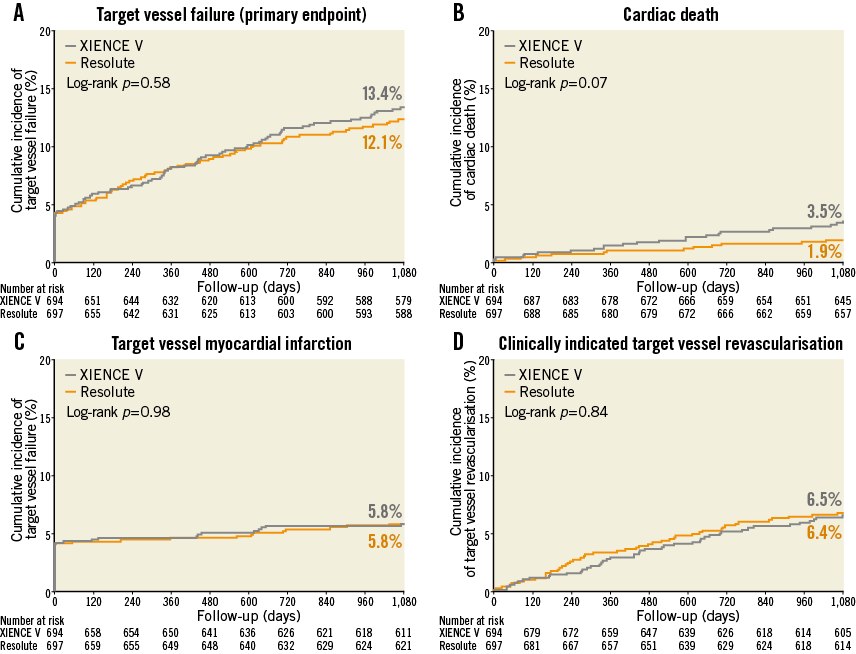
Figure 1. Kaplan-Meier curves for the composite endpoint target vessel failure (TVF) and its individual components until three-year follow-up. (A) TVF, a composite of cardiac death, target vessel-related MI and target vessel revascularisation; (B) cardiac death; (C) target vessel-related MI; (D) target vessel revascularisation. P-values were derived from the log-rank test; they may differ from p-values reported in the manuscript, which were derived from χ2 analysis.
x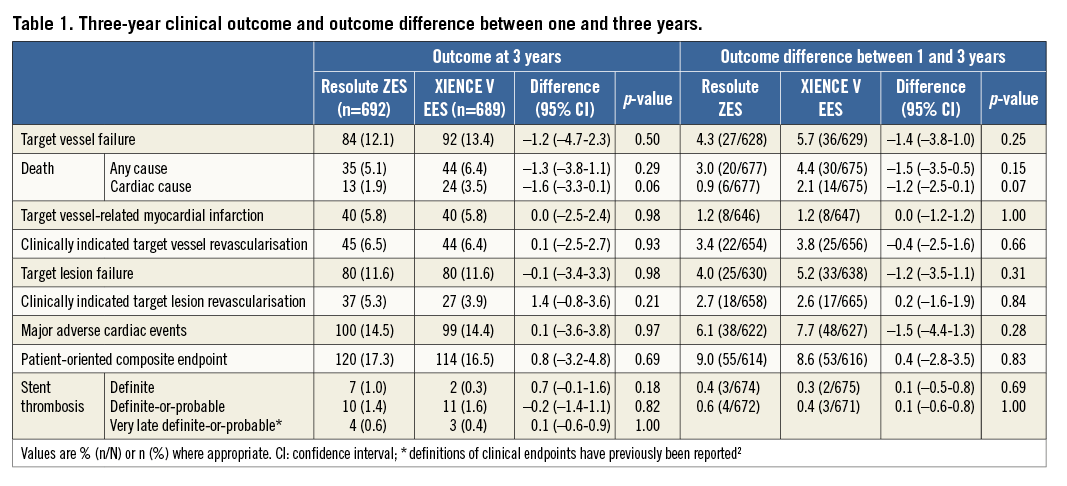
In addition, as shown in Table 1 and Figure 2, the rates of very late definite-or-probable stent thrombosis (>12 months) were similar and low (4/692 [0.6%] vs. 3/689 [0.4%], p=1.0). During three-year follow-up, the rates of definite stent thrombosis (7/692 [1.0%] vs. 2/689 [0.3%], p=0.18) and definite-or-probable stent thrombosis (10/692 [1.4%] vs. 11/689 [1.6%), p=0.82) remained low and similar for both groups. At three-year follow-up, 5.0% (33/657) of patients in the Resolute ZES group and 5.9% (38/645) of patients in the XIENCE V EES group were on dual antiplatelet therapy (acetylsalicylic acid plus P2Y12 inhibitor).
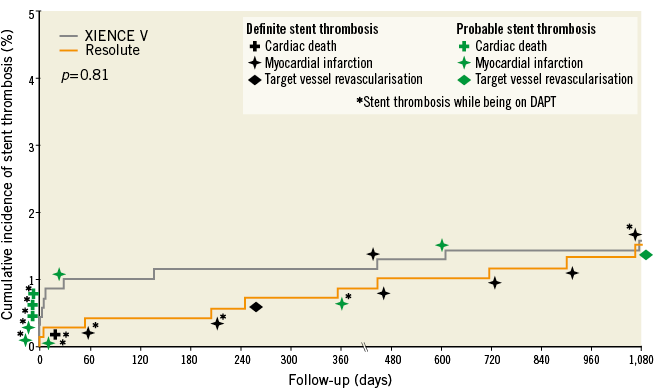
Figure 2. Cumulative incidence of definite-or-probable stent thrombosis at three-year follow-up. The cumulative incidence of definite-or-probable stent thrombosis, according to the Academic Research Consortium definition, is shown. DAPT: dual antiplatelet therapy (i.e., acetylsalicylic acid plus P2Y12 receptor antagonist)
There were also no statistically significant differences in the rates of other individual clinical endpoints such as target vessel-related MI (40/692 [5.8%] vs. 40/689 [5.8%], plog-rank=0.98), clinically indicated TVR (45/692 [6.5%] vs. 44/689 [6.4%], plog-rank=0.84) and cardiac death (13/692 [1.9%] vs. 24/689 [3.5%], plog-rank=0.07; Figure 1). The Kaplan-Meier curves of cardiac death tended to diverge after one year. We therefore performed post hoc a one-year landmark analysis which showed no significant difference in cardiac death during the first year (7/695 [1.0%] vs. 10/692 [1.4%], plog-rank=0.46; HR 1.43, 95% CI: 0.55–3.77) and, beyond one year, there was only a statistically non-significant trend (6/677 [0.9%] vs. 14/675 [2.1%], plog-rank=0.08; HR 2.19, 95% CI: 0.83–5.77; patients who died during the first year were excluded).
Discussion
Only very limited long-term data of randomised trial populations with ≥ three-year follow-up are available for the second-generation Resolute ZES. The present three-year clinical outcome data of the TWENTE trial that were obtained from 1,381 (99.3% of all enrolled) patients corroborate the favourable long-term outcome in the RESOLUTE All Comers trial, which is the only other randomised DES trial that reported ≥ three-year clinical outcome of Resolute ZES1. The observed trend towards a lower cardiac mortality in TWENTE patients treated with Resolute ZES cannot be explained by baseline or procedural data. It might be a play of chance. Nevertheless, further assessment of clinical follow-up of this study population is of interest.
This study was not powered to assess between-group differences in secondary clinical endpoints, such as cardiac death. In addition, our findings may not be generalised to patients with an acute ST-elevation MI (first 48 hours), as such patients were not enrolled in TWENTE.
Conclusion
The present three-year follow-up of the TWENTE trial demonstrates a similar and sustained safety and efficacy of the second-generation Resolute ZES and XIENCE V EES.
| Impact on daily practice The favourable three-year outcome data after use of both permanent-polymer drug-eluting stents (DES) in the broad study population of the TWENTE randomised trial, which comprised a real-world patient population with many patients who had complex lesions and various comorbid conditions, are a strong signal of sustained safety and efficacy of the compared devices in clinical practice. In addition, the consistently low rates of adverse clinical events such as stent thrombosis, target vessel-related myocardial infarction and repeat revascularisation, which were similar for both DES, represent an important benchmark for future comparison with long-term outcome data to be obtained after the use of novel DES and biodegradable scaffolds in complex patients. |
Funding
The investigator-initiated TWENTE trial had been supported by equal unrestricted grants from Abbott Vascular and Medtronic. The present three-year follow-up of patients randomised in TWENTE has been supported by a grant from Medtronic.
Conflict of interest statement
C. von Birgelen is or has been consultant to and has received lecture fees or travel expenses from Abbott Vascular, Boston Scientific, and Medtronic; he received travel expenses from Biotronik and lecture fees from MSD; the institution has received research grants from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. All other authors have no further conflicts of interest to declare.

