Abstract
Aims: We report here the final 5-year follow-up results from the ENDEAVOR II trial, which was the first randomised trial evaluating the Endeavor™ zotarolimus-eluting stent (ZES) compared with a bare metal stent (BMS) in patients with single, de novo coronary artery lesions.
Methods and results: Eligible patients were randomised 1:1 to receive ZES or BMS and were followed by telephone or clinic visit up to five years. We evaluated TVF and its components (target vessel revascularisation [TVR], Q-wave or non Q-wave myocardial infarction, or cardiac death attributed to the target vessel) at five years. Additionally, we report rates of MACE, TLR, and stent thrombosis (protocol- and ARC-defined) through five years. ENDEAVOR II enrolled 1,197 patients (598 ZES, 599 BMS). At five years of follow-up, the rates of TVF (15.4% vs 24.4%), TVR (10.7% vs 20.1%), MACE (15.4% vs 24.6%), and TLR (7.5% vs 16.3%) remained significantly lower in ZES patients compared with BMS patients. ARC definite and probable very late (>1 year) stent thrombosis remained low (0.2% ZES and 0.3% BMS) through five years.
Conclusions: After five years of follow-up, ZES demonstrated significantly improved clinical outcomes with sustained safety compared with BMS in patients with obstructive coronary artery disease.
Introduction
In-stent restenosis is a significant complication associated with the use of bare-metal stents (BMS). The recognition of this important adverse event led to the development of drug-eluting stents (DES) that inhibit smooth muscle cell proliferation and extracellular matrix production. These devices have successfully reduced the restenosis rates observed with BMS1-8.
Despite the improvement in restenosis rates with DES as compared to BMS, evidence has emerged of very late stent thrombosis (after one year) with some DES5,7,9-12. The antiproliferative properties of some DES may delay or prevent vessel healing, which has been proposed as a contributing factor to the development of late stent thrombosis. New stent technologies have been developed using novel antiproliferative drugs, polymers and delivery systems that are designed to improve biocompatibility, reduce hypersensitivity reactions, and extend drug elution, with the ultimate goal of reducing late stent thrombosis13.
It has not yet been proven whether these advancements will reduce the risk of very late stent thrombosis. Long-term follow-up is essential for any new stent technology to evaluate the risk of late stent thrombosis and associated clinical events. The ENDEAVOR II trial was a prospective, randomised, double-blind, multicentre trial of the Endeavor™ zotarolimus-eluting stent (ZES) (Medtronic CardioVascular, Santa Rosa, CA, USA) versus a Driver BMS (Medtronic CardioVascular, Santa Rosa, CA, USA). The primary endpoint and clinical outcomes through 24 months have been reported4. Use of a ZES reduced the risk of angiographic restenosis at eight months, as well as reduced target vessel failure (TVF), target lesion revascularisation (TLR) and overall major adverse cardiac events (MACE) at nine months as compared to the BMS. MACE and TLR rates remained significantly lower for patients receiving the ZES compared with the BMS at 24 months and there were no documented protocol-defined late stent thromboses4. ENDEAVOR II followed patients annually through five years post-procedure. The purpose of this analysis is to report the final 5-year follow-up outcomes from the ENDEAVOR II trial.
Methods
Overview and study population
A full description of the ENDEAVOR II methodology and results up to two years has been published4. Briefly, ENDEAVOR II was a randomised, double-blind, multicentre trial of the ZES versus a BMS. The study was conducted at 72 sites in Asia/Pacific, Australia, Europe, Israel and New Zealand in compliance with the Declaration of Helsinki. The protocol was approved by local ethics committees, and all participants provided voluntary, written informed consent.
Eligible patients included those with clinical evidence of ischaemia or a positive functional study who were undergoing stenting of a single, de novo lesion in a native coronary vessel with a reference vessel diameter of 2.25 to 3.5 mm and a lesion length ≥14 mm and ≤27 mm. Key exclusion criteria included any of the following: left ventricular ejection fraction <30%; >50% stenosis proximal or distal to the target lesion; myocardial infarction (MI) within the previous 72 hours; contraindications or allergy to aspirin, heparin, clopidogrel, cobalt, nickel, or chromium; hypersensitivity to contrast media; serum creatinine >2.0 mg/dL (177 µmol/L); leukocyte count <3000 cells/mm3; platelet count <100,000 or >700,000 cells/mm3; interventional coronary procedure within 30 days before or planned after implantation of the study stent; left main or ostial target lesion; severe calcification by angiography; bifurcation lesion; or location of target lesion at a >45° bend.
Study endpoints
The primary endpoint of ENDEAVOR II was the 9-month rate of TVF, comprising target vessel revascularisation (TVR), recurrent Q-wave or non Q-wave MI, or cardiac death attributed to the target vessel. This analysis reports the 5-year follow-up for the primary endpoint and its components, the rates of MACE (death, MI, emergent cardiac bypass surgery, or TLR), and stent thrombosis (protocol- and Academic Research Consortium [ARC]-defined).
Study procedures
Participants were randomised 1:1 to either ZES or BMS. All patients received a clopidogrel loading dose of 300 mg, at least 75 mg of aspirin and unfractionated heparin to maintain an activated clotting time >250 seconds (or 200-250 seconds if a glycoprotein IIb/IIIa inhibitor was used). Stents were implanted according to standard procedures; the specific procedural methods have been reported4. Aspirin ≥75 mg daily was prescribed indefinitely and clopidogrel 75 mg/day was prescribed for 12 weeks. Follow-up occurred at 30 days, six months, nine months and annually thereafter for five years.
Angiographic follow-up at eight months was specified for the first 600 consecutive patients enrolled and for all patients implanted with two or more stents.
Device overview
The Driver BMS is a cobalt alloy, low profile stent with a strut thickness of 0.0036 in (91 µm). The Endeavor ZES stent system consists of the BMS coated with phosphorylcholine and 10 µg zotarolimus per 1 mm stent length. The polymeric phosphorylcholine coating mimics the predominant phospholipid in the red blood cell outer membrane and has high biovascular compatibility4. Both stent types were delivered using a rapid exchange delivery system.
Statistical analysis
The intent-to-treat population (all patients in whom a study stent implantation was attempted) was used for this analysis. Categorical discrete variables were presented as counts and percentages and were compared by the χ2 test or the Fisher exact test when appropriate. The important clinical endpoints were also presented by Kaplan Meier estimates and were compared by log rank test. Continuous variables are presented as mean±standard deviation and were compared with the Student t test. All probability values are 2-sided.
Results
Baseline characteristics
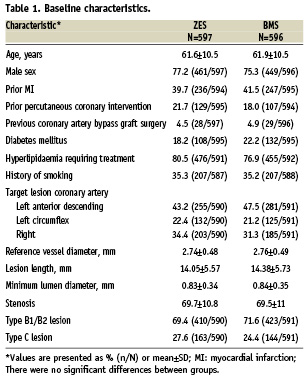
A total of 1,197 (598 ZES, 599 BMS) participants were enrolled between July 14, 2003, and January 13, 2004. Four (one ZES, three BMS) of these participants were randomised but did not undergo a procedure; therefore, the intent-to-treat population included 1,193 participants (597 ZES, 596 BMS).
Baseline characteristics are shown in Table 1. The mean age was 62 years, the majority of patients were male (76.3%), and diabetes mellitus was common (20.2%). A history of MI was present in 40% of the ZES group and in 42% of the BMS group. Approximately 20% had a prior percutaneous coronary intervention (PCI), and one-third had unstable angina. The target lesion was most commonly located in the left anterior descending artery, followed by the right coronary artery and the left circumflex. There were no between-group differences in any baseline clinical or lesion characteristic.
Clinical outcomes
At five years, follow-up was available for 577 of the 598 (96.7%) participants randomised to the ZES and for 582 of 596 (97.7%) participants randomised to the BMS. At five years, participants randomised to receive the ZES had a significantly lower rate of MACE, TLR, TVR, and TVF compared with participants randomised to the BMS (Table 2, Figure 1). At year one and year five of follow-up, patients who received a ZES had lower event rates compared with those who received a BMS for all clinical outcomes (Table 2) with cumulative incidence curves for efficacy favouring ZES (TLR, Figure 1A) and a trend for safety (ARC definite and probable stent thrombosis) showing early divergence of ZES from BMS although this is not statistically significant (Figure 1C).
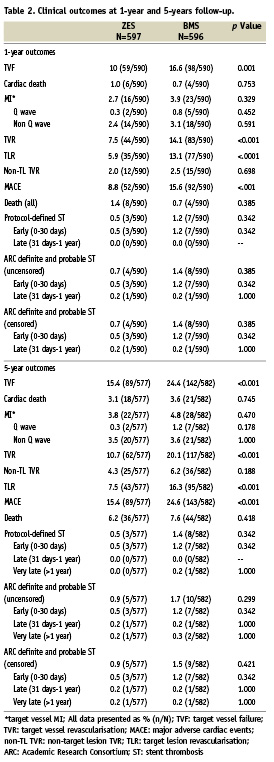
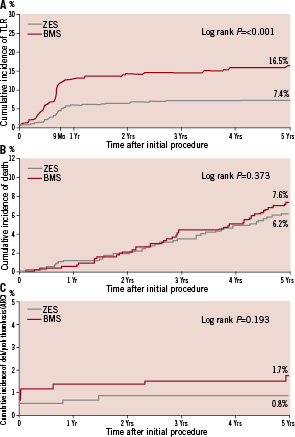
Figure 1. Cumulative incidence of clinical events to five years. Cumulative incidence curves at five years for zotarolimus-eluting stents (ZES) and bare metal stents (BMS) for (A) target lesion revascularisation, (B) all-cause death, and (C) definite and probable stent thrombosis.
There were very few cases of very late stent thrombosis between years one and five (Table 2). ARC definite and probable stent thrombosis was reported in one of 577 (0.2%) patients in the ZES group and two of 582 (0.3%) patients in the BMS group (p=NS). At five years, 88.5% of patients with a ZES were taking aspirin, 14.7% were taking clopidogrel, and 8.5% were taking dual antiplatelet therapy with aspirin and clopidogrel; among patients with a BMS, 86.5% were taking aspirin, 12.7% were taking clopidogrel and 8.6% were taking dual antiplatelet therapy (DAPT) (Figure 2).
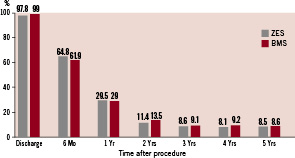
Figure 2. Dual antiplatelet therapy use to five years. Rates of dual antiplatelet therapy for patients receiving a zotarolimus-eluting stent (ZES) or a bare metal stent (BMS) at baseline, six months and annually to five years. DAPT: dual antiplatelet therapy.
Discussion
The ENDEAVOR II trial was designed to evaluate the long-term efficacy and safety of the ZES compared with a BMS in a population of patients with single-vessel coronary artery disease. The 5-year results from ENDEAVOR II demonstrated a low rate of clinical events (TVF, TLR, TVR, and MACE) with sustained efficacy for participants randomised to the ZES. Furthermore, this trial demonstrated significant superiority in terms of prevention of restenosis, and there was no late catch-up restenosis associated with the ZES. Very late (>1 year) stent thrombosis remained low at 0.2% through the 5-year follow-up period, and no evidence of other safety concerns emerged over time.
This sustained safety profile may be related to improved biocompatibility of the ZES as compared to earlier stent systems. Delayed healing secondary to antiproliferative properties has been implicated as a potential factor predisposing DES to a higher risk of stent thrombosis14,15. The ZES phosphorylcholine polymer coating provides an outer phospholipid layer that mimics the outer membrane of the red blood cell thus enhancing stent biocompatibility16 and reducing thrombogenicity17. Zotarolimus was the first cytostatic drug designed exclusively for rapid elution from the phosphorylcholine polymer stent coating18 and was shown to significantly inhibit neointimal formation in the porcine coronary model19. Whether these properties of the ZES translate to lower rates of stent thrombosis and associated clinical events, will be evaluated in the PROTECT trial, in which 8,800 ‘real world’ patients will be randomised between the ZES and the sirolimus-eluting stent (SES) (Cypher, Cordis Corporation, Miami, FL, USA)20.
These results showed that there were no protocol-defined late stent thrombosis events in ENDEAVOR II. The rate of very late ARC definite and probable stent thrombosis was also very low, with only one event in the ZES group and two events in the BMS group during this entire period despite the declining use of DAPT at one year and thereafter (Figure 2). The observed symmetry in antiplatelet therapy between study arms neutralises a potential positive confounding effect of longer antiplatelet therapy on stent thrombosis, assuming an equal effect on ZES as BMS, and facilitates the interpretation of the outcomes. Low rates of very late ARC definite and probable stent thrombosis with ZES have also been observed in other ENDEAVOR trials. The ENDEAVOR III trial compared ZES with SES and at five years reported rates of 0.7% for ZES and 1.0% for SES (p=0.756) with only 0.34% ARC definite and probable stent thrombosis after year one21. In a recent pooled analysis of over 2,100 patients receiving a ZES there was an ARC definite and probable stent thrombosis rate of 0.8% at five years with only 0.18% after year one. The rate of DAPT use in these patients was 7.5% at five years22. The Danish Organisation for Randomised Trials with Clinical Outcome (SORT-OUT) III comparing ZES with SES in an all-comers population reported no difference in definite stent thrombosis at 18 months (1% for both, p=0.13), although other cardiac events (MI, TVR and TLR) were lower at nine months for the SES group23. Patients were prescribed DAPT for one year post-procedure although actual DAPT adherence at one year is not reported. It is unclear why events rates in patients receiving the SES were lower than observed in other clinical trials using this DES but outcomes for ZES were consistent with the observed low 2-year TLR rate of 5% and definite and probable stent thrombosis rate of 0.7% in the E-Five Registry which also included a high proportion of complex patients24. In other studies of comparable size comparing a DES versus a BMS in patients with a single de novo lesion, the rate of very late stent thrombosis was 0.8% for SES in the SIRIUS study, 1.7% for SES in the RAVEL study and 2.2% for the paclitaxel-eluting stent in the TAXUS IV study25-27. The percentage of patients on long-term DAPT is not available for the SES studies. The TAXUS IV trial reports that 31% of the patients were receiving DAPT at five years. Additionally, the five year rate of TLR with SES was 9.4% in the SIRIUS study and 10.3% in the RAVEL study. TAXUS IV reported 9.1% TLR at five years. The low TLR rate in at five years in the present study of 7.5% further supports the long-term safety and efficacy of the Endeavor ZES.
The 2006 guidelines for percutaneous coronary intervention recommend that clopidogrel administration be continued ideally up to 12 months after DES implantation in patients who are not at high risk of bleeding28. The low rate of very late stent thrombosis combined with the low rate of dual antiplatelet use after one year observed in this trial make it difficult to assess any relationship between long-term DAPT and very late stent thrombosis events. While there is no definite proof that extending the duration of DAPT beyond six months or one year is desirable after DES implantation in all cases, many patients are currently prescribed thienopyridines for longer periods of time in the hope that this precautionary approach might prevent some late thrombosis events. Whether this good intention translates into improved outcomes is currently being investigated in large prospective trials29.
In the current study, the same proportion of patients in the BMS and ZES groups received a DES in response to observed in-stent restenosis. Details of specific stent type are not available for all patients; however, the comparison of censored and uncensored thrombotic events showed no difference in rates of ARC definite and probable stent thrombosis at five years. This observation suggests that secondary stent thrombosis (after intervening TLR) has a low incidence and is unrelated to the original type of stent (DES or BMS) implanted. However, because of the limitations of this analysis (post hoc, low number of restenosis events, low number of secondary stent thrombosis); a larger study is needed to confirm this observation.
Stent thrombosis is a relatively rare event but warrants further clinical research from both an individual patient perspective because of the serious clinical sequelae, usually death or myocardial infarction, as well as from a society perspective because of the large number of coronary stents implanted yearly. In the current trial, the ZES arm had a rate of stent thrombosis similar to that of the BMS control arm, but the study limitations (relatively small sample size and selected patients with a single de novo lesion) should be acknowledged.
Conclusion
Based on these extended follow-up data from ENDEAVOR II, the ZES stent is associated with a sustained efficacy and safety profile and very low rates of stent thrombosis out to five years as compared to the BMS. After one year, the revascularisation rates plateau; consistent with the overall ENDEAVOR program results. These findings are encouraging and suggest that the ZES stent is a safe and effective device for the management of patients with coronary artery stenosis.
Acknowledgements
The authors gratefully acknowledge the following individuals for project management support of this study: Yvonne Groot from Quintiles and Judith Mosterd, MSc, and Frank van Leeuwen, MD, from Medtronic. Assistance with the development of this manuscript was provided by CommGeniX, LLC.

